 |
Low Vision: Concepts and Clinical Skills for Generalists
Learn how to comprehensively assess the visual status of your patients and coordinate their care with an appropriate specialist.
By Erin Kenny, OD, and Christin DeMoss, OD
Release Date: January 15, 2021
Expiration Date: January 15, 2024
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Assess visual status in various disease categories.
- Review staging of different diseases and the likely visual prognoses.
- Discuss how vision outcomes have changed with newer treatment options.
- Prepare patients for the low vision journey.
Target Audience: This activity is intended for optometrists engaged in the care of patients with vision loss from ocular disease.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Erin Kenny, OD, Salus University; Christin DeMoss, OD, Salus University.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 70642-LV. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: Drs. Kenny and DeMoss have nothing to disclose..
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
Vision CareCheck out the other feature articles in this month's issue: -Where Does Vision Care Fit in 2021? |
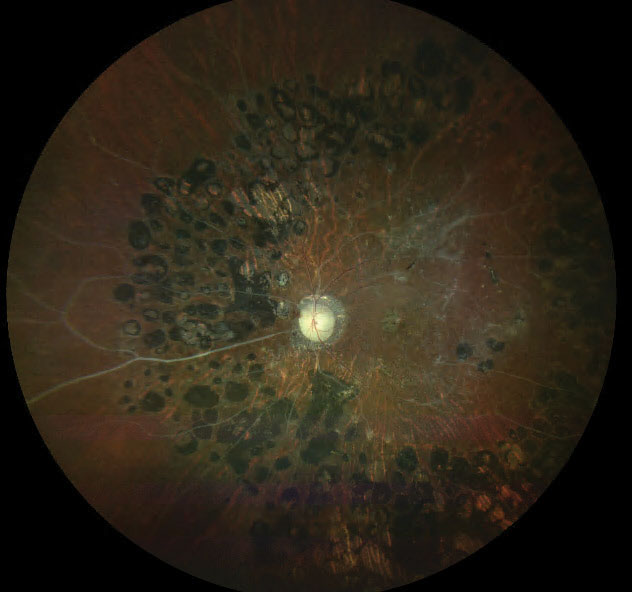 |
| Fig. 1. This widefield fundus photograph demonstrates sclerotic blood vessels, optic nerve pallor and retinal laser scarring in a patient with a history of proliferative diabetic retinopathy status post panretinal photocoagulation treatment. Click image to enlarge. |
Given the vital role vision plays in every aspect of life, all optometrists—regardless of specialty—must be able to distinguish between visual problems that can be corrected with conventional interventions and those that cannot. Low vision, which interferes with everyday activities, cannot be fixed with glasses, contact lenses, or other standard treatment approaches.1 Therefore, it is critical that primary care optometrists understand how to support these patients in their practice, even if their role is limited to assessment and comanagement.
This article will provide an update on the pathophysiology behind low vision, how it manifests clinically and the decision-making process a primary care optometrist can use to determine what can be managed in your practice and what requires referral to a low vision specialist.
Ocular Pathology Review
Let’s review the top causes of persistent visual deficits with an eye toward understanding how they present.
Diabetic eye disease. In 2019, the International Diabetes Federation estimated that about 10% of the global population—approximately 463 million people—are living with diabetes.2 Diabetic eye disease, which includes diabetic retinopathy and diabetic macular edema, is the most common microvascular complication of diabetes.3 Of the two types of diabetes, diabetic retinopathy is more commonly seen in patients with
Type 1 diabetes. The literature suggests up to 90% of patients with Type 1 diabetes will develop proliferative diabetic retinopathy after 30 years.4
Optometrists play an integral role in detecting and managing diabetic eye disease and, in many cases, are the first to detect the condition. They should not only be familiar with determining the stage of diabetic retinopathy, but also with the possible functional vision side effects, even in cases where acuity is not affected. Functional vision side effects are often not reported until the appearance of macular edema or following retinal laser therapy (Table 1).
Many times, the sight preserving treatments of diabetic eye disease result in the most profound functional vision complaints. Current treatment options for proliferative diabetic retinopathy include retinal laser and intravitreal anti-VEGF injections. Treatment options for non-proliferative diabetic retinopathy, include central laser photocoagulation (grid, focal) and intravitreal anti-VEGF injections. Even when visual acuity improves after these treatments, patients will likely experience permanent functional vision impairments.
The greatest functional vision impairment is commonly seen in patients with a history of proliferative diabetic retinopathy with panretinal photocoagulation. This includes:
–Visual field constriction: missing/tripping on curbs or stairs and many times do not feel comfortable in unfamiliar environments, difficulties with continuous reading, unable to localize objects in their environment.
–Impaired contrast sensitivity: difficulties with reading or activities of daily living.
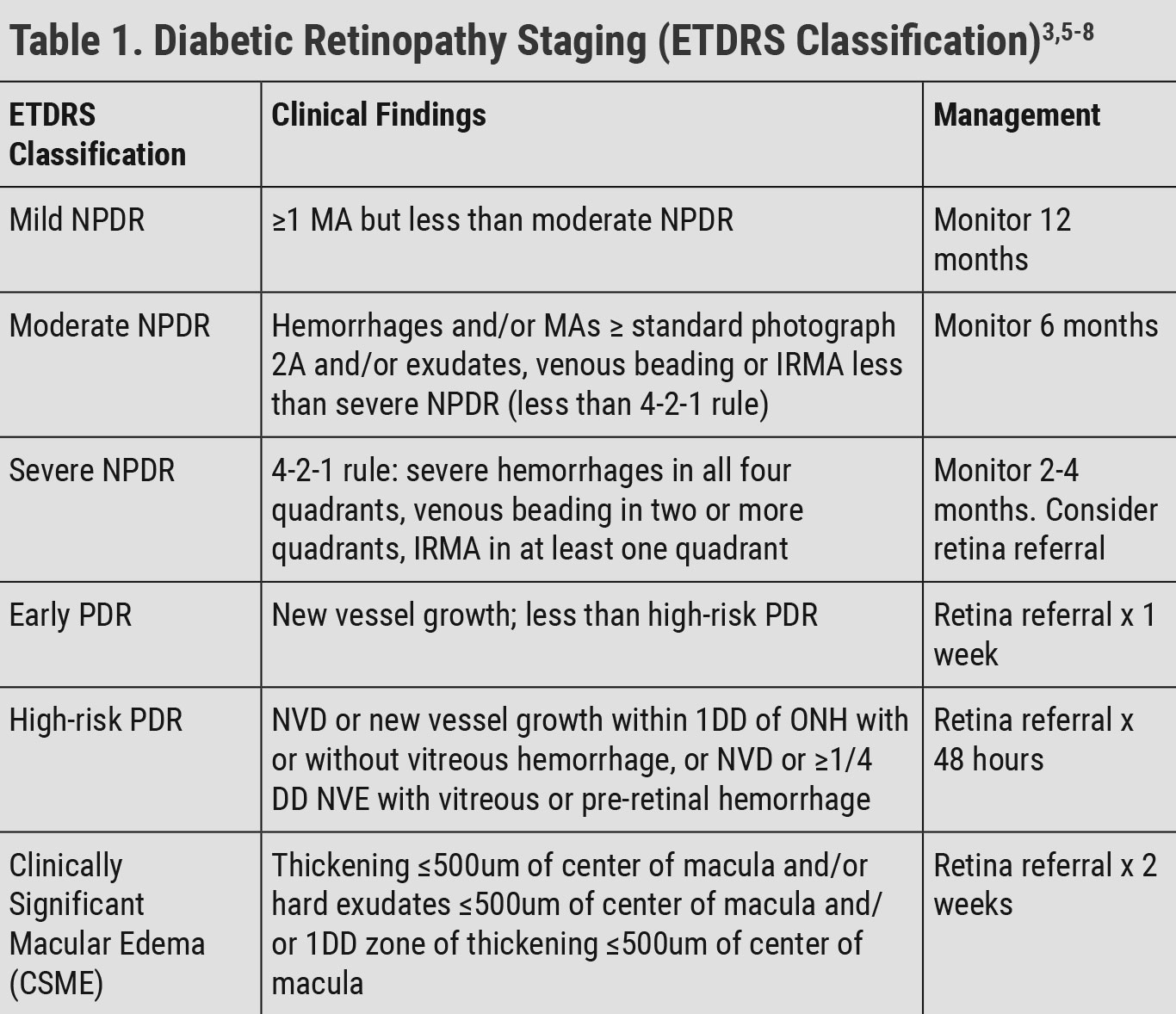 |
| NPDR: non-proliferative diabetic retinopathy, PDR: proliferative diabetic retinopathy, MA: microaneurysm, IRMA: intraretinal microvascular abnormality, NVD: neovascularization of the disc, DD: disc diameter, NVE: neovascularization elsewhere. Click table to enlarge. |
Age related macular degeneration (AMD). This is the leading cause of irreversible blindness in individuals over 50 years of age.9 The prevalence of AMD increases with age and is highest in individuals over the age of 80.10 AMD is a chronic, progressive disease characterized by the accumulation of extracellular debris known as drusen between the RPE and Bruch’s membrane.10,11 While much remains unknown about AMD, the different stages and indicated treatments are well documented (Table 2).
There are two main forms, non-exudative and exudative, with non-exudative AMD accounting for about 90% of all AMD cases. Risk factors for AMD include age, Caucasian race, positive smoking history, genetics, systemic hypertension and history of excessive sunlight exposure.11
Optometrists are key providers in diagnosing and managing early AMD and comanaging late-stage AMD. It is important to understand functional vision implications of this condition, which include:
–Central scotoma: common in late-stage disease, patients may complain of missing lines or losing their place while reading, patients may eccentrically view during distance and/or near vision testing, distance acuity and near reading acuity may not correlate.
–Dark adaptation impairment: especially earlier in the disease course, patients may complain of difficulties with adjusting to drastic changes in lighting, patients with previous history of asymptomatic photochromatic lens wear may report lenses not adjusting quickly enough.
–Contrast sensitivity impairment: patients may report difficulties with reading or activities of daily living.
Glaucoma. This group of ocular disorders is defined by progressive, permanent optic nerve cupping with corresponding visual field defects and retinal ganglion cell loss. The most common types of glaucoma include primary open angle and primary angle-closure.13 Glaucoma currently affects nearly 2.25 million Americans ages 40 and older with 1.6 million of those individuals experiencing significant visual impairment.14 It is estimated to be the second leading cause of irreversible blindness in the United States and is the leading cause of irreversible blindness in black and Hispanic individuals.13,14
Optometrists play an integral role in the treatment and management of glaucoma with topical and, in some states, surgical interventions. They frequently make referrals for surgical interventions but may not always recognize the functional vision implications of glaucoma.
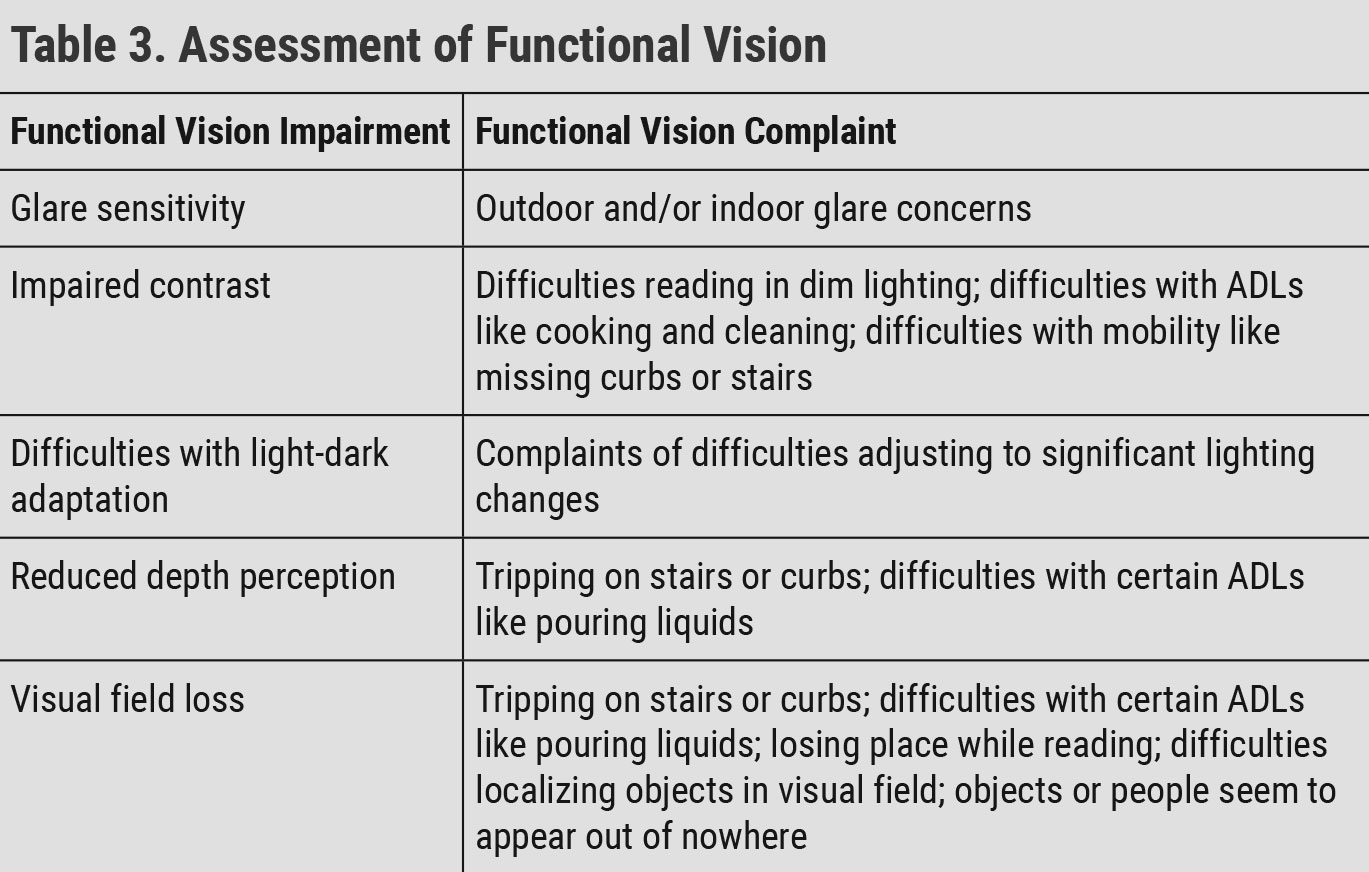
As eye care providers, we should be aware of the functional vision impairments that correspond with each stage of glaucoma. Mild glaucoma can result in increased glare sensitivity, impaired contrast sensitivity and impaired light-dark adaptation. Functional vision impairments associated with moderate stage disease include increased glare sensitivity, impaired contrast sensitivity, reduced depth perception and visual field loss. Patients with advanced glaucoma may have increased glare sensitivity, impaired contrast sensitivity, reduced depth perception, visual field loss and decreased visual acuity (Table 3).
While some patients with glaucoma report functional vision complaints early on in the disease, many do not report problems until the disease reaches advanced stages. Eye care providers should probe patients for the functional vision complaints listed above and make the appropriate recommendations or referral(s) to address their patients’ concerns.
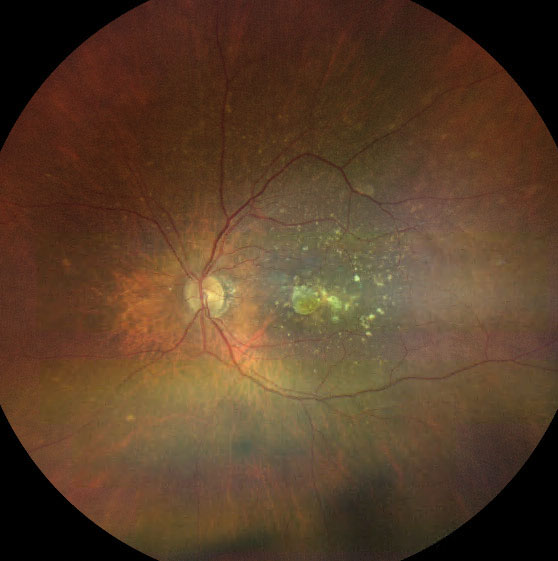 |
| Fig. 2. Late AMD with large confluent drusen and a small area of central geographic atrophy. Click image to enlarge. |
Night blindness. Nyctalopia can be an exclusive disorder or the result of another ocular pathology. Disorders such as retinitis pigmentosa (RP), choroideremia, gyrate atrophy and congenital stationary night blindness are all inherited retinal dystrophies that result in nyctalopia. Treated proliferative diabetic retinopathy with panretinal photocoagulation can also cause night blindness, as well as vitamin A deficiency, zinc deficiency and uncorrected myopia. For the purpose of this article, we will focus on RP and congenital stationary night blindness.
RP is one of the most common inherited retinal disorders, with a prevalence of one in 3,500 to 4,000 in the United States and Europe.15 Early stages of the disease result in night blindness and mid-peripheral field loss, specifically a ring scotoma. This ring scotoma can cause mobility issues but also reading and near work problems. As the disease progresses, the ring scotoma spreads out peripherally before it starts to move in to affect central vision. Late stages result in central vision loss and may result in complete blindness. RP is a pathology where rods are affected first, hence why it is defined as a rod-cone dystrophy (RCD). These dystrophies present with retinal atrophy and pigment clumping in the periphery, and eventually maculopathies in the later stages.
Clinical presentation typically depends on the inheritance pattern of the disease.16 Generally, autosomal recessive RP presents with night blindness and severe vision loss earlier in life in comparison to autosomal dominant inheritance, which has a more gradual onset in adulthood and has visual sequelae that are less severe.17 The most severe results come from the x-linked recessive inheritance pattern. These patients have a similar clinical presentation in onset and severity as autosomal recessive.20 Additionally, there are sporadic mutations as well, which can result in varying severity. Genetic testing and pedigrees can help determine the inheritance pattern of RP.
Congenital stationary night blindness is an inherited retinal dystrophy that occurs at birth. Patients present with normal visual fields and it can present as incomplete or complete forms. Once again, genetic testing can be beneficial to determine the type and severity of the disease. Complete forms will show a more severe defect in the rod photoreceptor signal transmission and, as a result, worse night vision or rod response in ERGs. This disorder is not progressive and will remain stable throughout life, therefore, lacking “stages” that we see in other pathologies. Mobility and lighting should be discussed with these patients due to their ocular complaints.
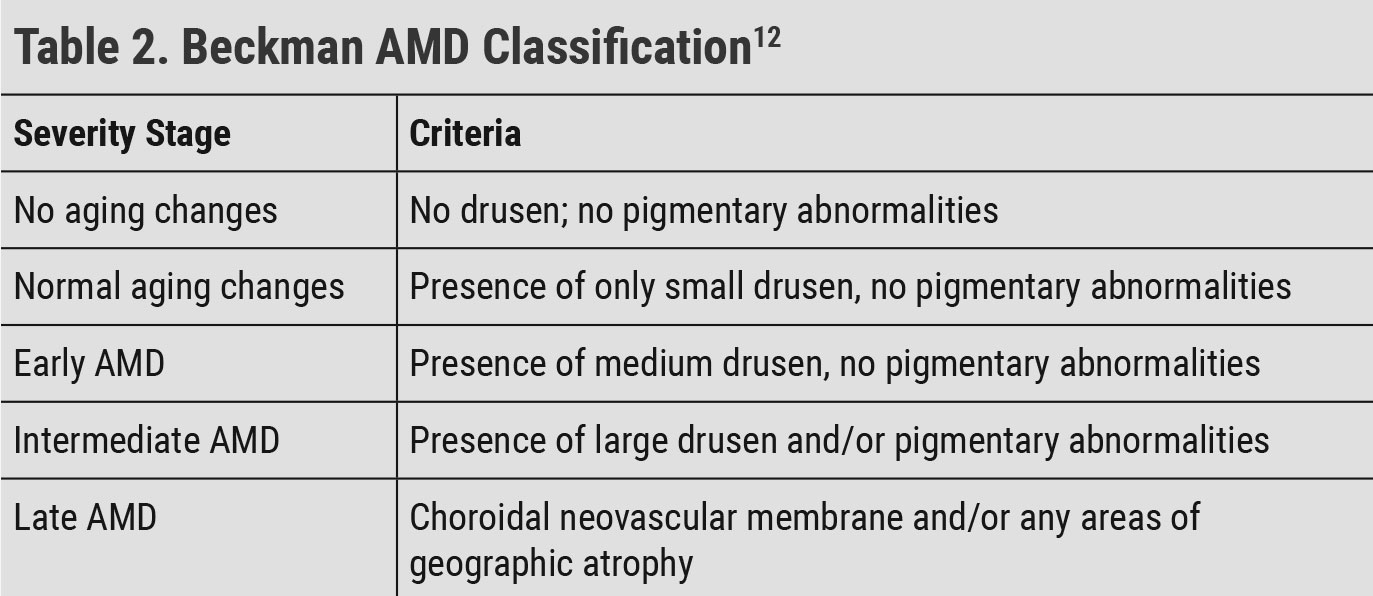 |
| Pigmentary abnormalities: any hyper- or hypopigmentation not associated with other retinal disease. Small drusen: ≤63µm. Medium drusen: ≥63µm and ≤125µm. Large drusen: >125µm. For reference, 125µm = diameter of a retinal vein at optic nerve head. Click table to enlarge. |
Color deficiency and day blindness. A variety of ocular disorders can result in color deficiencies and photosensitivity for our patients, including optic neuropathies, maculopathies and neurological disorders. Color deficiency can also present as a benign, inherited disorder. Red-green color blindness, which is made up of varying protanopes, deuteranopes, proton anomalies and deuteranomalies, affects nearly 8% of males.18 Let’s now review the prognosis and staging of achromatopsia and cone-rod dystrophies.
Achromatopsia is a disorder that can present in the complete (typical) or incomplete (atypical) form. The distinguishing points between the two types is dependent on the residual cone function. The complete form of achromatopsia results in decreased visual acuity around 20/200 and severe photophobia and light sensitivity by six months old.18 It is not a progressive disorder but often results in hemeralopia, also known as day blindness. Complete achromatopsia results in the inability to distinguish colors at all. Incomplete achromatopsia can result in better acuities (as good as 20/80) and less significant clinical findings due to partial cone function. In the incomplete form, patients may be able to discern between shades of grey and cognitive recognition of these “colors.” Both forms of achromatopsia benefit from red tints (whether in spectacles or contact lenses) since the rod is the main functioning photoreceptor. It should be noted that the fundoscopic examination of these patients frequently show no evidence of the disease, but a central scotoma is often present on visual field examination.
Cone dystrophies, along with cone-rod dystrophies (CRD), usually present in the first three decades of life.19 CRDs and RCDs will have the same end result of severe vision loss, but the timing of onset of the photoreceptor dysfunction is what differentiates them. CRDs usually present earlier in life and result in central vision loss, dyschromatopsia, light sensitivity and central scotomas before the disease progresses to affect the rods and cause night blindness.
Most CRDs are broken into two stages. Stage 1 is known for a decreased central visual acuity which results in a “noticeable deviation of gaze to project images on parafoveal regions of their retina that are less damaged.”20 Stage 1 also consists of severe photophobia and different levels of color deficiency. ERG findings show the cone responses are significantly more impaired than rod responses. Funduscopic findings may include pigment deposits and macular atrophy. Temporal optic nerve pallor may be noticed due the papillomacular bundle being affected. During this stage, light sensitivity should be evaluated with a variety of tints and filters and magnification should be explored due to the central blur. Stage 2 of CRD shows a continuing decrease in visual acuity, along with the additional night blindness and loss of peripheral field as a result of the rods being affected.20 Due to the loss of both rods and cone function of RCD and CRDs, these patients often need extensive vision rehabilitation.
Low Vision Management
ICD-10 coding defines low vision as best corrected visual acuity ranging from worse than 20/70 to 20/400 or better in the affected eye(s) and blindness as worse than 20/400 (Table 4).21 Although ICD-10 coding defines low vision and blindness this way, optometrists using vision measurements beyond visual acuity are aware that a patient can complain of functional vision difficulties well before their vision meets the ICD-10 guidelines. Low vision rehabilitation is not reserved solely for patients with low vision or blindness based on ICD 10 guidelines and should be considered for patients with any acuity with functional vision concerns.
Seeing Beyond "Acuity"Vision is the culmination of a multi-step process where light rays enter the eye and are converted into electrical signals by the retina to be processed by the brain. Relying on a singular measure like visual acuity for a complex process provides only a one-dimensional measurement of vision. Taking advantage of multiple measurement techniques allows for a more comprehensive overview of all aspects of a patient’s vision. As optometrists, we should be knowledgeable about all measurement systems to best care for and manage the visual status of our patients. This includes, but is not limited to, visual acuity, peripheral visual field, central visual field, glare sensitivity, color vision and contrast sensitivity. All of these components can be affected throughout the varying stages of different ocular conditions. With the right examination techniques and awareness, primary care optometrists can identify and provide patients with simple recommendations or the appropriate referral. |
Optical and non-optical visual function management. After collecting the information about a patient’s functional vision, it is important to be knowledgeable and realistic about the devices and services available. While it is imperative to provide patients with hope regarding low vision rehabilitation, it is just as important to avoid unrealistic expectations. One of the most common errors is stating that a low vision provider can simply offer glasses to help a patient see better. Referring doctors should instead educate patients that low vision providers maximize a patient’s remaining vision or provide alternatives such as text-to-speech devices to turn nonfunctional vision into functional vision. It also prevents the patient from focusing on a “magic” pair of glasses.
Low vision devices are categorized into working distances: near, intermediate and distance. Near devices can help patients with near-related tasks, such as reading, writing or threading a needle. Intermediate devices can help patients with that working distance consistent with using a computer or reading music while playing an instrument. Distance devices can be useful when a patient needs to spot signs or see the television better. In combination with non-optical approaches, optical devices are a great way to help a patient achieve their functional vision goals.
There are a number of near and intermediate optical devices available. For example, microscopes, which are spectacle mounted convex lenses, are simply a high add power. They can be set in a variety of ways, including full fields, prism half eyes or even high add bifocals. Microscopes are defined by any add power that is over +4.00 diopters. Another near device that is often recommended and familiar to patients is a handheld magnifier. This tool is the same convex lens that is used in a microscope but placed on a handle. This allows the magnifying lens to be held at a greater distance away from the eye. Handheld magnifiers often provide illumination with a built-in light which can be beneficial to many of our patients with impaired contrast.
Stand magnifiers are another near magnification option. These devices are a plus lens that is mounted at a particular distance from the reading material on a stand. These can be helpful for patients who do not want to hold a device and are not interested in the close working distance a microscope often requires. A telemicroscope is a device that can be used at intermediate distances as well as near. It is the combination of a telescope and a microscope. Video magnification is another optical device utilized for near magnification. They come in a variety of forms, including portable and desktop video magnification. These devices are beneficial for patients who may require magnification, increase in contrast and a larger field of view.
Primary care optometrists can consider providing simple optical and non-optical aids for patients with mild visual impairment. The main concept used for low vision near optical devices is equivalent power. Determining one functional power for a patient’s specific goal is a quick and easy way to make simple device recommendations without referral:
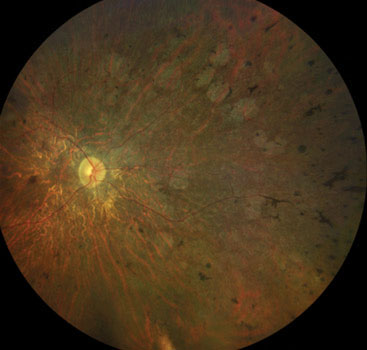 |
| Fig. 3. A late-stage retinitis pigmentosa patient with severe retinal atrophy, bone spicules and attenuated vessels. Click image to enlarge. |
Step 1: Determine your patient’s visual goal (e.g., reading, writing, sewing)
Step 2: Assign an acuity needed to perform that goal:
- 8pt font is equivalent to 1M sized print, which equals 20/50 vision at 40cm
Step 3: Set up a proportion using your patient’s best corrected near reading vision and goal vision
- Patient’s near vision OU is 2M at 30cm (0.3/2M) with a +3.00 add
- Patient’s goal vision OU is 1M
- Proportion: 0.3/2M = x/1M, x = 0.15cm, D = 1/0.15cm = +6.75
Step 4: Provide your patient with a +6.75D add to read 1M (8pt font) at 15cm
It is important to note that if a threshold near vision acuity is used instead of a reading near vision acuity, the equivalent power calculated will only help the patient spot read the goal line, not read comfortably. It is recommended to always start the equivalent power calculation with reading acuity for this reason.
A telescope is the only optical device that can be utilized for improvement of distance acuity. There are two categories of telescopes: handheld or spectacle mounted. In addition, the only two types of telescopes we use in low vision are Keplerian and Galilean. Depending on the patient’s goals, a low vision doctor will recommend the appropriate setting of each device. For example, a patient with low magnification needs (e.g., visual acuity is 20/80) may use a handheld Galilean telescope to accomplish the goal of spotting street signs (e.g., a target goal of 20/40).
Primary care providers can consider recommending telescopes for patients who need distance magnification. Determining the appropriate telescopic power is simple:
Step 1: Determine your patient’s visual goal (e.g., street signs, TV)
Step 2: Assign an acuity needed to perform that goal
- 20/40 is a great starting point for street signs
Step 3: Determine the magnification needed
- Patient’s distance vision OD/OS/OU is 20/120
- Patient’s goal vision 20/40
- Patient’s vision/goal vision = 120/40 = 3x
 |
| ADL: activity of daily living. Click table to enlarge. |
Head-mounted technology (HMT) offers magnification, increased contrast, different polarity options and different field of view options to benefit patients with varying ocular diseases. HMT has its role in low vision but should not be considered the end-all solution for every patient. While each low vision device arguably needs some level of rehabilitation or training, HMT devices in particular require significant training to ensure patient success. Primary care providers should be aware these devices exist and can educate patients on the device capabilities; however, they should consider referring to a low vision provider for device determination and training.
Smartphones and tablets are one of the most significant advancements the low vision industry has seen in recent years. These devices are readily accessible to many patients and offer numerous vision accessibility features. Adjustable features include enlarged font, increased contrast, different polarity options, text to speech and virtual assistants. In addition, free and low-cost downloadable applications can provide functions such as optical character recognition (text-to-speech), color identification and money identification. The new functional history question all patients are now asked is: “do you have a cell phone and if so, what kind is it?” The patient’s response can alter our starting point for showing optical devices. Primary care providers can absolutely offer recommendations for phone and tablet accessibility features and applications.
Non-optical solutions. In addition to simple optical device and smartphone/tablet recommendations, non-low vision optometrists should consider making suggestions regarding lighting. Another simple low vision rehabilitation concept is the perpendicular light concept. Two bulbs of equal wattage and equal distance to a page will provide different levels of illumination depending on the angle at which the bulb is held. Bulbs held perpendicular will provide greater illumination. Additionally, the closer the bulb to the page, the greater the illumination. A 75-watt bulb held five feet away will provide less illumination than a 50-watt bulb located two feet away.
Simple recommendations that can be made to patients to increase contrast and reading acuity include purchasing a gooseneck desk lamp and floor lamp that can be manipulated to provide direct, perpendicular illumination on the reading material. Recommending lighting that is positioned away from the patient’s eyes is also important to reduce complaints of glare sensitivity.
In contrast to lighting, sun lenses and tints can be beneficial for glare reduction and contrast enhancement. There is no hard and fast rule to which tint goes with each pathology. Rather, tints and sun lenses should be evaluated based on transmission and color. Depending on a patient’s needs and where the glare actually occurs, we can make recommendations for sunglasses, tint overlays, acetate filters and settings/accessibility adjustments. It is not uncommon for patients to have multiple tints depending on the different situations when they complain of glare.
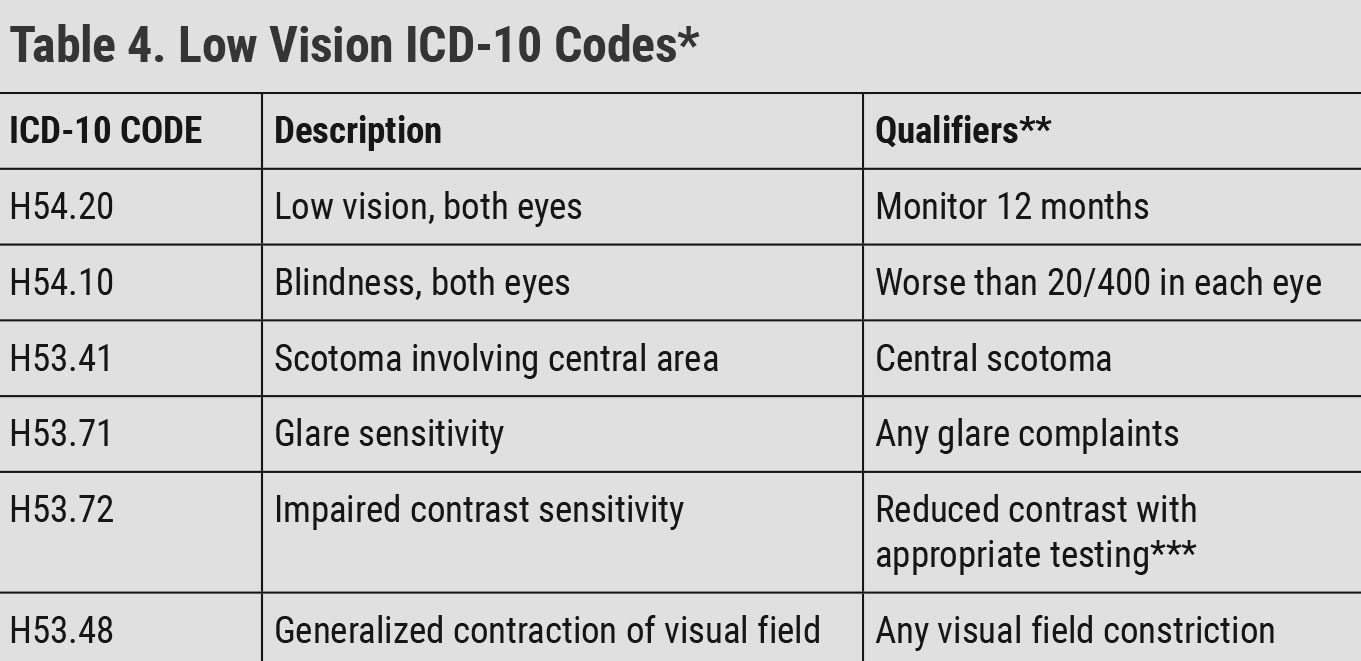 |
| *The codes in this table are only samples of general low vision codes. There are over 35 ICD-10 low vision codes. Reference the American Academy of Ophthalmology’s Vision Rehabilitation Preferred Practice Pattern for the full set of low vision codes.22 **World Health Organization change to definition of blindness document.21 ***MARS Contrast Sensitivity, Pelli-Robson Contrast Sensitivity, LEA Contrast Sensitivity. Click table to enlarge. |
Field enhancement. Although most providers summarize a patient’s vision as central visual acuity, a clinician must not forget about the options we can provide for our patients with visual field loss. With regards to field loss, prism is the most common device for visual field enhancement. It should be reiterated to patient’s that they will not regain their lost visual field, but we will enhance their remaining field.
Sectoral Fresnel and Eli Peli prisms are tools that can be used with a patient who has a hemianopsia. Sectoral prisms work by instructing the patient to scan into the prism placed on the side of the patient’s defect. The prism will allow the image to be shifted into the field that they are able to use. Eli Peli prisms, on the other hand, are placed above and below the patient’s pupil. This allows the patient to have an early warning of objects on the side of their defect without having to scan to the side of their field loss. Yoked prism can also be used for reading in a patient with hemianopsia. This type of prism does not expand the field but rather shifts the image, allowing less head turn to be needed to read. For patients with concentric field loss from pathologies, such as RP and glaucoma, a reverse telescope may be an option. This tool minifies the image the same amount of the magnification of the telescope. For example, a 2x telescope, when reversed, will enhance the patient’s visual field by two times. However, the patient’s central acuity will also be reduced by two. All field enhancement devices should be finalized for functionality with a rehab specialist.
Psychosocial aspects and additional referrals. Functional vision loss, no matter what stage, can affect a patient’s entire life. Psychosocial concerns can include job jeopardy, depression/anxiety, difficulties with connections in the community, difficulties at school, inability to drive and overall decreased quality of life.
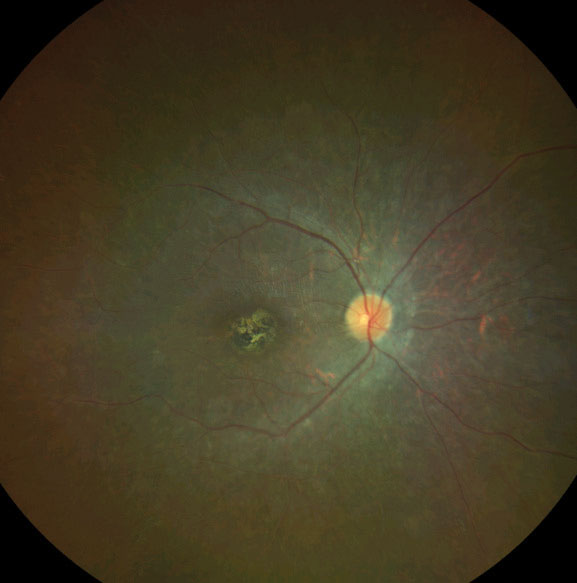 |
| Fig. 4. This photo shows a cone-rod dystrophy with overall retinal atrophy and pigmentary changes in the foveal region. Click image to enlarge. |
A patient who is suffering from functional vision loss may also benefit from providers that are not optometrists, such as orientation and mobility specialists, vision rehabilitation therapists, certified low vision therapists, and counselors. State agencies (lowvision.preventblindness.org/us-orgs/#p) are a great starting point to help these patients get connected to specialists they may need.
To Sum Up
Optometrists are at the forefront of managing a wide variety of ocular disorders with varying visual acuities and stages such as diabetes, macular degeneration and glaucoma. They should be just as knowledgeable about the low vision treatment options and recommendations for each stage of these disorders as they are with the current pharmaceutical and surgical options.
Dr. Kenny is currently the chief of the William Feinbloom Vision Rehabilitation Center and an assistant professor at Salus University. She has lectured on the national and international level with a focus on vision rehabilitation.
Dr. DeMoss is a clinical instructor at Salus University’s Pennsylvania College of Optometry and The Eye Institute. She assists with the low vision rehabilitation didactic course and sees patients in both the primary care and low vision service at The Eye Institute. Neither author has any financial interests to disclose.
| 1. National Eye Institute. Low Vision. www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/low-vision. 2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th Edition. Diabetes Res Clin Pract. 2019;157:107843. 3. Cheloni R, Gandolfi S, Signorelli C, et al. Global prevalence of diabetic retinopathy: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9(3):e022188. 4. Bowling B, Kanski JJ. Kanski’s Clinical Ophthalmology : A Systematic Approach. 8th ed., Edinburgh, Elsevier, 2016;520-537. 5. No authors listed. Early treatment diabetic retinopathy study design and baseline patient characteristics. Ophthalmology. 1991;98(5 Suppl):741-56. 6. Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677-82. 7. Wong T, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608-22. 8. Flaxel, Christina J., et al. Diabetic Retinopathy Preferred Practice Pattern. Ophthalmology. 2020;127(1):P66-P145. 9. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606-17. 10. Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;(59)2:74-7. 11. Bowling B, Kanski JJ. Kanski’s Clinical Ophthalmology: A Systematic Approach. 8th ed., Edinburgh, Elsevier, 2016;599-615. 12. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-51. 13. Gupta D, Chen P. Glaucoma. Am Fam Physician. 2016;93(8):668-74. 14. Medscape. Primary open-angle glaucoma (POAG). emedicine.medscape.com/article/1206147-overview. 15. Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl. 2002;233:1–34. 16. National Eye Institute. Retinitis pigmentosa. www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinitis-pigmentosa. 17. Bagheri, N. (2017). Retinitis pigmentosa and inherited chorioretinal dystrophies. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. Philadelphia, PA: Wolters Kluwer; 2017:341-42. 18. Remmer MH, Rastogi N, Ranka MP, et al. Achromatopsia: a review. Curr Opin Ophthalmol. 2015;26(5):333-40. 19. Bagheri, N. (2017). Cone Dystrophies. In The Wills eye manual: Office and emergency room diagnosis and treatment of eye disease (pp. 346). Philadelphia, PA: Wolters Kluwer. 20. Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. 21. World Health Organization. Blindness and vision impairment. www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment. 22. Fontenot JL, Bona MD, Kaleem MA, et al. Vision rehabilitation preferred practice pattern. Ophthalmology. 2018; doi:10.1016/j.ophtha.2017.09.030. |
