These ocular structures are crucial to ocular health, and knowing the conditions that affect them will help optometrists better care for their patients.
By Lindsay Sicks, OD
Release Date: April 15, 2020
Expiration Date: April 15, 2023
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe the anatomy and function of the corneal nerves.
- Discuss the ways in which various pathologies disrupt corneal nerve function.
- Clinically assess corneal nerves.
- Review what treatment options are available.
Target Audience: This activity is intended for optometrists engaged in the care of patients with corneal nerve dysfunction.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Lindsay Sicks, OD.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 67777-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Sicks has received fees for non-CME/CE services from Alcon.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
| The Corneal Layers The cornea is an approximately 0.50mm thick avascular tissue with five distinct layers.6 The outermost epithelial layer provides a smooth refracting surface as well as a barrier against infection.62 Bowman’s membrane, which lies under the epithelium and its associated basement membrane, has a function that is unclear in the literature. Some postulate that it may act as a physical barrier to protect the subepithelial nerve plexus, thereby facilitating rapid stromal wound healing, recovery of anterior corneal transparency and restoration of epithelial innervation after trauma.63 The corneal stroma is the largest layer, accounting for 90% of the total corneal thickness. It is made up of intertwined fibrils of collagen lamellae that provide structure to the cornea. Descemet’s membrane and the single-layer corneal endothelium form the innermost layers. The endothelial cells employ a sodium-potassium-ATPase pump to maintain tissue clarity by achieving an appropriate level of corneal dehydration.17 |
Corneal nerve structure and function have been extensively examined both in vivo and ex vivo.1 A solid understanding of these factors will help clinicians recognize the signs and symptoms of corneal nerve damage and assist them in making the proper diagnosis. It will also help them initiate treatment, when appropriate, for ocular surface disease, ocular pain and corneal disease, infection or trauma.
Structure & Function
The corneal nerves begin to form at five months gestation in humans, as neural crest cells differentiate from the lateral border of the neural plate.1 This process is induced and regulated by several proteins that control the differentiation of the neural crest cells. Some of the cells develop into cranial neural crest cells, and among those derivatives is the trigeminal ganglion, a sensory ganglion of the trigeminal nerve.1 Also known as cranial nerve (CN) V, the trigeminal is the largest of the cranial nerves with three branches: the ophthalmic, maxillary and mandibular. Together they span the face up to the vertex of the scalp and cover the oral and nasal cavities. The ophthalmic and maxillary branches are purely sensory (e.g., touch, pain, temperature) while the mandibular branch also has a motor component. Recall that the branches of the ophthalmic nerve are the frontal, lacrimal and nasociliary branches.2
The cornea is the most densely innervated tissue in the human body, containing 70 to 80 large nerves and approximately 7,000 free epithelial nerve endings (nociceptors) per 1mm2.1,3 The tissue has been defined and characterized using light microscopy, electron microscopy and confocal microscopy.1
| Annual Cornea Report Check out the other feature articles in this month's issue: |
Most corneal nerve fibers arise from the ophthalmic branch of the trigeminal nerve, forming thick bundles that approach the cornea radially to form the limbal plexus.4,5 At 1mm to 3mm away from the limbus, the nerves lose their perineurium and myelin sheath, aiding in corneal transparency. These stromal nerves, encased in only Schwann cells, run at a mean depth of approximately 300µm from the corneal surface.1,6 Within the stroma, they extend laterally and anteriorly, running parallel to the collagen lamellae.3 Branches form the anterior stromal plexus and continue anteriorly to form the subepithelial nerve plexus (between Bowman’s layer and the anterior stroma), penetrate Bowman’s layer to form the subbasal nerve plexus and branch further to enter the corneal epithelium where they terminate.5-8
Corneal nerves play a role in the maintenance of a healthy cornea, including the blink reflex, wound healing and maintenance of the ocular surface. Nerves release neuromediators that provide nutritional support and elicit protective reflexes, such as tear production and blinking, in response to injury. When there is sensory nerve damage, corneal homeostasis mechanisms are affected and neurotrophic signaling is lost, negatively impacting corneal nerve function. The cornea could experience a reduction in epithelial cell turnover and blink rate as well as disruption of tear formation. As a result, the corneal epithelium releases neurotrophins—a family of growth factors related to nerve growth factor (NGF)—that help regulate the growth, proliferation, function and survival of the corneal epithelium, thereby maintaining a healthy ocular surface.4
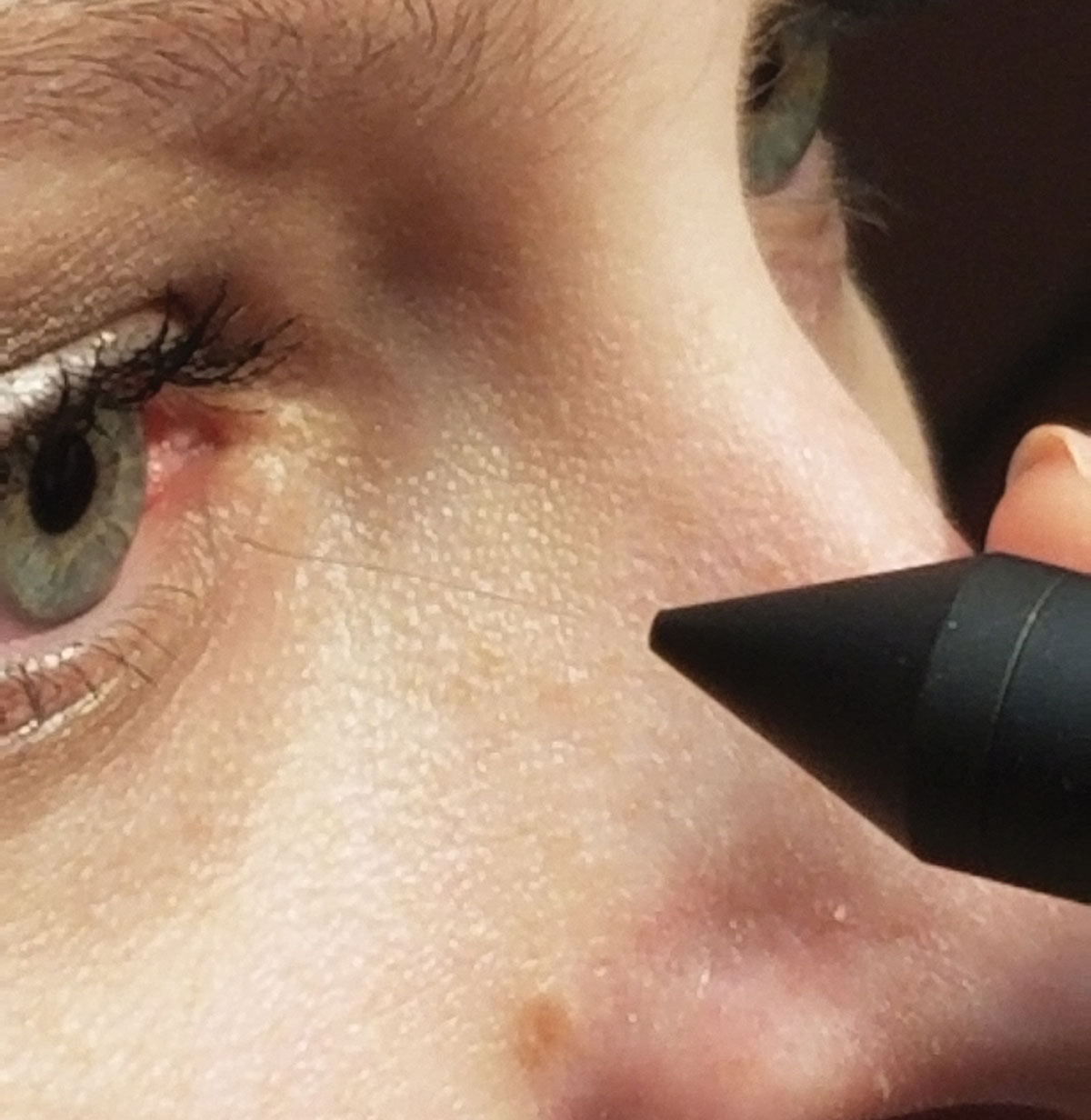 |
| Fig. 1. A Cochet-Bonnet esthesiometer is designed for rapid assessment of corneal sensitivity. Click image to enlarge. |
Clinical Assessment
Corneal nerve structure and function are adversely affected by many ophthalmic and systemic conditions.6,9,10 Therefore, it is critical to have tests for corneal nerve structure and function with good diagnostic capability.10 Several important advancements have emerged recently for imaging and assessing corneal nerves.
Corneal confocal microscopy. In vivo confocal microscopy (IVCM), invented in the 1950s, became widely used in the early 1990s.10-12 The imaging systems available today allow for high-resolution, real-time assessment of corneal nerve structure, including the epithelial nerves, subepithelial nerve plexus, subbasal nerve plexus and stromal nerves.1,9 The introduction of IVCM enabled imaging of the live subbasal nerve plexus, leading to the theory that nerve bundles are preferentially oriented in the superior-inferior direction at the corneal apex and in a nasal-temporal direction in surrounding areas.3
Imaging with IVCM requires the proper instrumentation, patients who will cooperate while images are obtained, expertise in the interpretation of confocal imaging and software tools for automated analysis.13 The tool is also limited in its ability to image the ultrastructure of various nerve bundles. If clinicians can obtain reliable images with IVCM, they can evaluate corneal nerve morphology and any abnormalities that occur due to various ocular and systemic diseases without altering the tissue microenvironment.1
Cranial nerve and corneal sensitivity testing. Since CN V is implicated in corneal nerve sensitivity, it is important to review the appropriate testing of the cranial nerves. During evaluation of the ophthalmic branch, a blink reflex upon corneal touch is expected. Clinicians could also test the patient’s forehead and/or scalp response to a light touch on each side, asking if both sides feel the same. To assess the maxillary branch, compare the touch response on the cheek or side of the nose. For the mandibular branch, compare touch on each side of the lower jaw.14
Current techniques for assessing corneal sensitivity have limited accuracy due to their subjective nature. The simplest involves touching the cornea gently with a wisp of cotton from a swab to initiate a blink response.6,15 The Cochet-Bonnet esthesiometer can provide a more formal measurement with quantitative results (Figure 1). It measures corneal sensitivity using a fine nylon filament that is introduced from the side and just touches the corneal surface. The longer the filament, the more flexible it is, indicating that more corneal sensation is present.6
Both of these approaches suffer from lack of diagnostic sensitivity and are generally disliked by patients.10 Non-contact (or “air puff”) instruments for corneal esthesiometry could be a potential improvement, but no such device to assess corneal nerves is currently commercially available. Further, such a device would likely require the patient to verbally confirm whether they felt the stimulus, introducing an element of subjectivity that may be undesirable.13,16
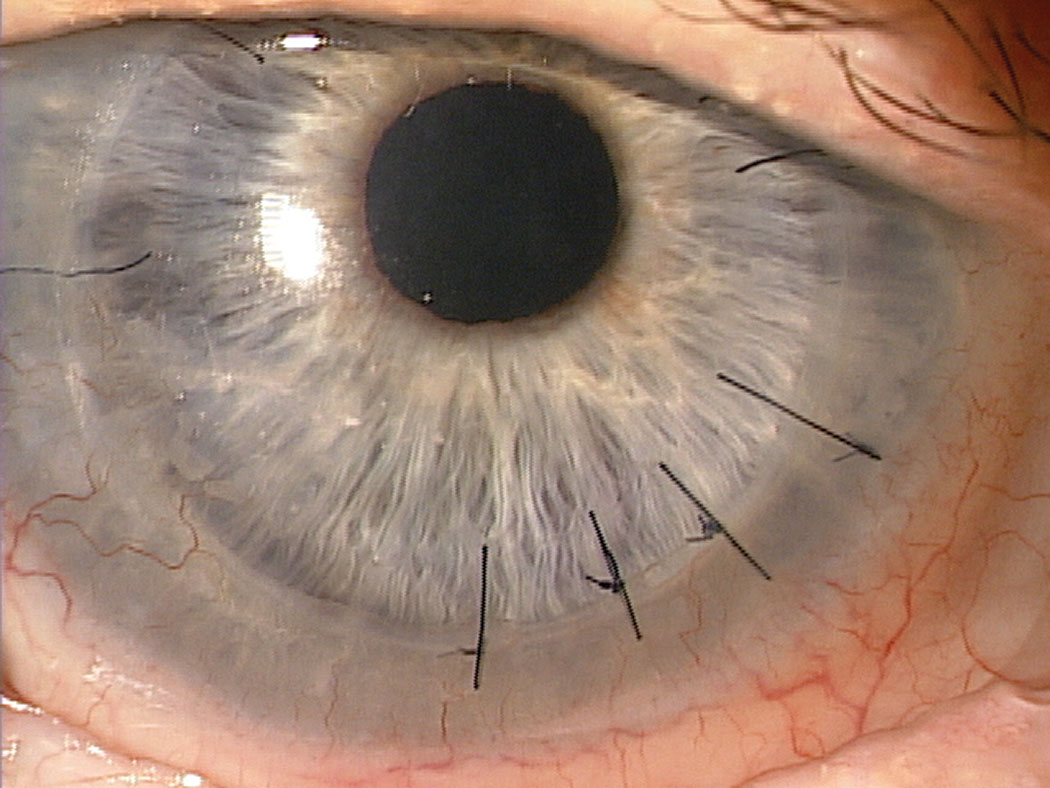 |
| Fig. 2. A full-thickness PKP with several interrupted sutures visible. All of the corneal nerves are transected when a PKP is performed. Photo: Jeffrey Sonsino, OD. Click image to enlarge. |
Researchers have proposed one novel approach to screening corneal sensitivity whereby hyperosmolar drops are instilled to cause a reflex eyelid squinting in rats.13 The more hyperosmolar the solution, the stronger the blink response. Applying the various hyperosmotic solutions caused an osmolarity-dependent increase in squinting of the treated eye in control rats. In contrast, the squinting response of diabetic rats to the most hyperosmolar solution was significantly reduced compared with healthy rats.13
The motor innervation of CN V affects the muscles of mastication. Testing for the temporalis and masseter muscle motor function involves palpation for symmetry while the patient bites down and clenches their teeth. Testing the pterygoid muscles involves having the patient move their jaw from side to side. A lesion of the motor fibers of CN V will result in asymmetry of muscle action or deviation of the jaw toward the weak side upon closing of the mouth.14
Corneal Nerve Disruption
Disruption of the normal corneal architecture by ocular or systemic disease can affect the structure and function of the corneal nerves.9,10
Post-surgical complications. Corneal surgical procedures lead to varying degrees of corneal nerve damage, and subsequent regeneration of these nerves takes time. For example, in the creation of a classic LASIK flap with a microkeratome, the superficial afferent sensory nerves in the anterior one-third of the stroma are transected.17,18 This disrupts ocular surface tear dynamics, resulting in symptoms of irritation and a reduction in corneal sensitivity.18,19 As a result, dry eye disease (DED) is the most common complication following laser refractive surgery and is correlated to the amount of preoperative myopia and the depth of laser treatment.20,21
Several possible mechanisms have been proposed for DED after LASIK, including the above-mentioned afferent sensory nerve damage during flap creation, a reduction in the blink reflex, reduced tear production, increased tear evaporation and injury to the goblet cells at the limbus.20
One study found the incidence of post-LASIK DED was less with the newer femtosecond laser–created flaps (9%) than with a traditional microkeratome (46%).22 Flap thickness does not correlate with dry eye symptoms, suggesting that other factors are important in the pathophysiology of LASIK-induced DED. One explanation for the decrease in dry eye with a femtosecond flap is that there is less damage incurred by the corneal nerves during flap creation and less damage to the limbal stem cells and goblet cells from the femtosecond fixation ring.20,23
The stromal nerves will usually reinnervate the cornea within five to eight months after LASIK surgery, improving dry eye symptoms.17,20,23 During a penetrating keratoplasty, all of the corneal nerves are cut, so the recovery of innervation may be slower (months to many years) or even non-existent.24
Patients may also experience corneal nerve dysfunction after a neurosurgical procedure (brain, spinal surgery, etc.) during which the trigeminal nerve is damaged. Research shows that 2.8% of patients undergoing surgical intervention for trigeminal neuralgia develop neurotrophic keratitis (NK).25,26
Penetrating keratoplasty. A full-thickness penetrating keratoplasty has been the standard of care for replacing diseased and compromised corneas.6 The procedure requires transection of all corneal nerves in both the host and donor cornea (Figure 2). Penetrating keratoplasty eyes can have marked central anesthesia or hypoesthesia for at least 18 months and up to 32 years following corneal transplantation.17,24,27 Factors such as age, preoperative diagnosis, contact lens wear, diabetes and elapsed time since surgery had no correlation with the timeline for return of sensitivity to the graft.24
Diabetes. This disease can affect a wide variety of corneal nerve characteristics. In animal studies, diabetic rats had reduced corneal nerve density in the subepithelial layer and reduced cornea mechanical sensitivity by esthesiometry. Both motor and sensory nerve conduction velocity and total nerve fiber length in the subepithelial layer were also significantly decreased in diabetic rats.13
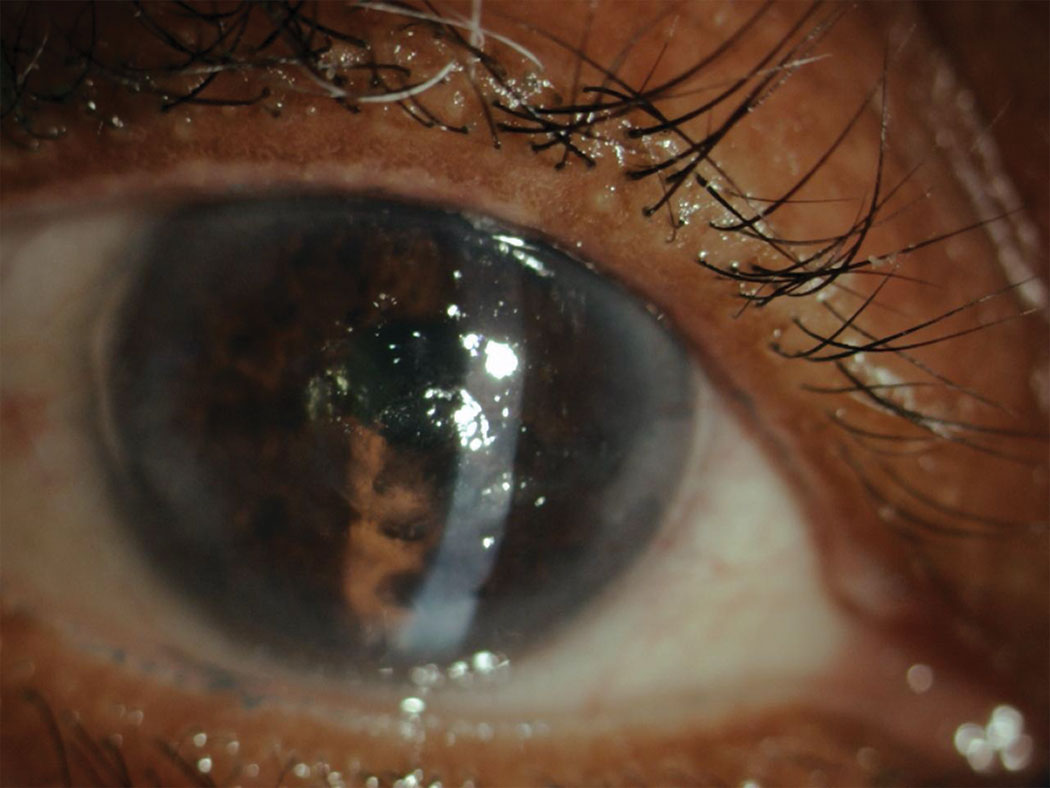 |
| Fig. 3. This African-American female patient developed HSV-associated neurotrophic keratopathy. Photo: Jennifer S. Harthan, OD. Click image to enlarge. |
Diabetic NK is not well-described in the literature. However, the effects of diabetes on the corneal nerve structure are better understood. The degree of reduction in corneal sensitivity in diabetic patients often correlates to the severity of diabetic neuropathy.28,29 A reduction of corneal nerves in diabetes patients may be the sole presenting feature of diabetes or may present concomitantly with proliferative diabetic retinopathy.30 Conversely, through the use of confocal imaging, improvement in corneal nerve morphology can be seen when risk factors for diabetic neuropathy improve.31
Infection. Herpetic viral infection, either simplex or zoster, is a leading cause of neurotrophic keratitis (Figure 3).32-34 After herpetic infection, the corneal nerves can undergo a loss of sensory fibers and a deficient or abnormal reinnervation process. Following herpes simplex keratitis, affected eyes have reduced nerve density, reduced total nerve count, reduced corneal sensation and the nerves themselves undergo regression.35,36 The corneal nerves can also be altered after other forms of corneal infection, such as Acanthamoeba and fungal infections, where central subbasal corneal nerve density and nerve fiber length are significantly diminished and there is a marked decrease in nerve branching.32,36
Further studies are needed to investigate whether these nerve changes are caused directly by the virus or indirectly by the elicited immune response and resulting inflammation; however, some research does suggest a role for interleukin factors in the inflammatory response.37 One study suggests corneal nerve alterations may be even more pronounced in Acanthamoeba and fungal infections than in herpetic infections.32
Dry eye disease. A study examining corneal nerves in patients with non-Sjögren’s dry eye found reduced corneal sensitivity, changes in nerve morphology and reduction in nerve density with confocal imaging in the dry eye group compared with the control group.38
In select patients with DED, suspicion should remain high for early NK. To ensure prompt diagnosis, clinicians should conduct a thorough case history with emphasis on past herpetic infection, diabetes, surgery or trauma. Upon clinical exam, the presence of punctate epithelial erosions and/or epithelial irregularities should prompt clinicians to consider the addition of corneal sensitivity testing, confocal imaging or both, if available. In the absence of such technology, clinicians must carefully monitor patients who do not respond to standard therapy, as it raises the clinical suspicion for NK.
Neurotrophic keratitis. This rare degenerative corneal epithelial healing disorder arises after denervation of the corneal surface.33 It is estimated that the prevalence of NK is fewer than five in 10,000 individuals and may be as low as 1.6 in 10,000.25,39-41 There are fewer than 65,000 people affected in the United States.42
All of the conditions already described here can lead to neurotrophic keratitis, as can chemical burn, radiation, corneal injury and corneal trauma. Various types of intracranial space-occupying lesions or related surgical treatments affecting trigeminal innervation to the cornea can also be a cause of NK.25
The clinical presentation of NK includes an array of signs and symptoms such as painless blurry vision, punctate keratitis, tearing, light sensitivity, epithelial thinning, increased epithelial permeability, neovascularization, persistent epithelial defect, corneal edema, corneal scarring and corneal ulceration (Figure 4).6,33,39
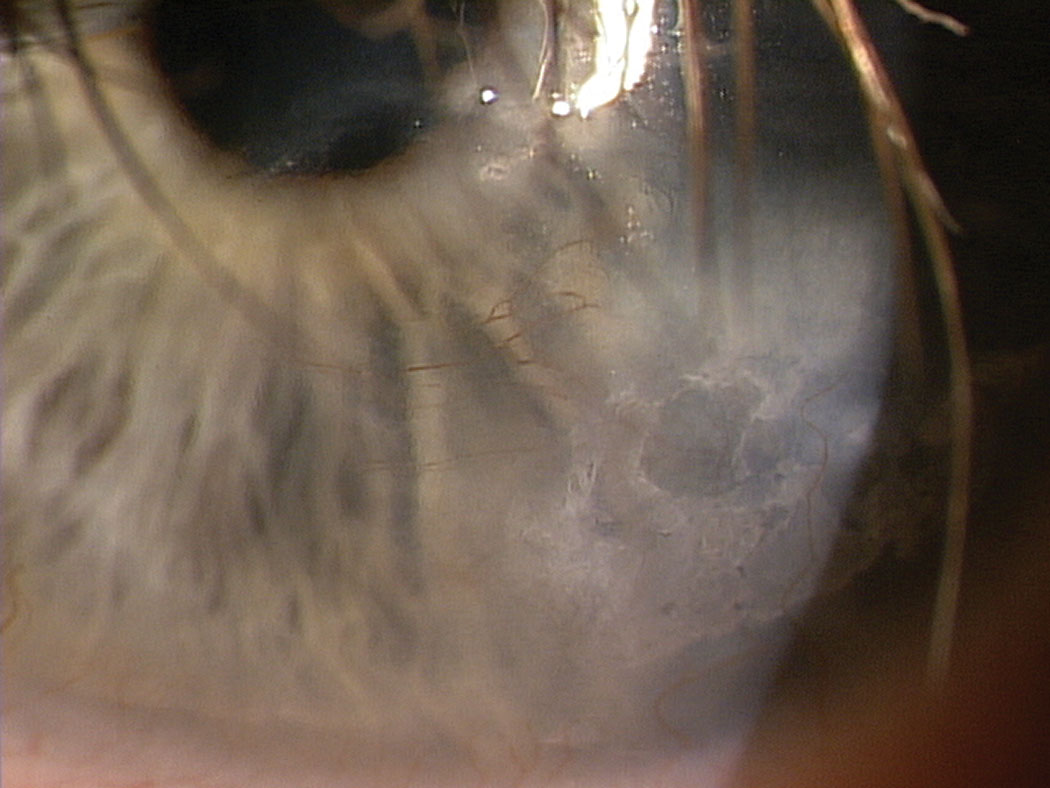 |
| Fig. 4. Here, neurotrophic keratitis presents with epithelial irregularity, corneal scarring and neovascularization. Photo: Jeffrey Sonsino, OD. Click image to enlarge. |
NK is typically described as a persistent, non-healing, epithelial ulceration that can progress to corneal melt and perforation in severe cases.39 Patients can be asymptomatic at advanced stages due to reduced corneal sensation. There is a risk of superinfection with bacteria or fungi and a risk of corneal melt precipitated by inappropriate use of topical steroid medications.33,43 Comorbidities, such as exposure keratitis, DED or limbal stem cell deficiency can negatively influence the outcome of NK and require prompt treatment.25
NK is classified into three overlapping stages based on severity.25,39 Staging is useful because some interventions are based on stage, and prompt treatment may halt progression to the next stage.43
Stage 1 (mild): Corneal epithelial irregularity, superficial punctate staining, mild stromal scarring, corneal edema and neovascularization.
Stage 2 (moderate): Persistent epithelial defect, Descemet’s folds, stromal swelling and possible anterior chamber reaction.
Stage 3 (severe): Corneal ulceration with stromal thinning that can progress to stromal melting, perforation or both.39
Restoring Function
Following clinical assessment, there are a number of treatment approaches to consider when addressing disruptions to the structure and function of the corneal nerves.
Contact lenses. Bandage soft contact lenses (BCLs) have many functions in the treatment of corneal disease, including protecting the ocular surface from exposure. A bandage lens decreases necrosis and desquamation of the corneal epithelium by prevention of blink-associated mechanical stress, allowing for subsequent acceleration of wound healing.44,45 In the treatment of NK, a BCL can prevent the need for tarsorrhaphy, with autologous serum dosed over the top, or in conjunction with cyanoacrylate glue to prevent corneal perforation.25,45
Scleral lenses have also been used in the treatment of refractory cases of DED and NK, as they can provide a tear reservoir for healing and contribute to corneal protection and hydration and present an alternative to tarsorrhaphy.46,47
Amniotic membranes. Applying one or more layers of amniotic membrane (AM), the innermost layer of the placenta, can be effective for the treatment of DED, herpetic infection and NK. In one study of NK, more than more than 75% of patients achieved re-epithelialization in 16 days while another study found an average time of 21 days.48,49 AM procedures have traditionally been performed in the operating room, but sutureless amniotic membranes (SAMs) are now available for in-office application.50
| Managing NK Patients Management of NK typically involves a stepwise approach depending on disease severity and staging. Stage 1 treatment consists of preservative-free tear supplementation, removal of any offending agents, prolonged patching and/or the addition of topical cyclosporine, lifitegrast or autologous serum tears.42 Topical steroids are controversial—their use may increase the risk of corneal melt and perforation secondary to upregulation of collagenases. At stage 2, BCLs, scleral lenses or amniotic membranes may be added.46,47 Stage 3 may warrant the addition of debridement or punctal occlusion as well as consideration of more invasive procedures such as a tarsorrhaphy or Gundersen flap.33 Surgical options are typically considered off-label and palliative in nature.42 An ideal treatment would go beyond palliative care and also stimulate epithelial healing, provide trophic support for the corneal tissue and restore sensation to the corneal nerves.42 Autologous serum and medications containing NGFs, such as Oxervate, have the potential to provide some of these benefits.55 Two randomized controlled multicenter, double-masked trials of 151 patients demonstrated complete corneal healing in 70% of patients treated with this drug. The recommended dosing schedule for Oxervate is six times daily for eight weeks. Potential adverse reactions include eye pain (16%), conjunctival hyperemia, eye inflammation and eye irritation.40,59 The eye pain and irritation could be associated with patients regaining corneal nerve function that was lost due to NK.60 |
Autologous serum. These are non-allergenic, non-preserved drops derived from blood serum—the component of blood that remains after clotting. Research speculates that the biochemical and biomechanical similarity of autologous serum to natural tears is inherent to its utility in the treatment of ocular surface disorders.51 Several tear factors are important in the maintenance of the corneal and conjunctival epithelium, such as epidermal growth factor, vitamin A, transforming growth factor β, fibronectin and other cytokines.45 In contrast, commercial artificial tear substitutes are typically optimized solely for their biomechanical properties.
Autologous serum can be useful in the treatment of DED, graft-vs.-host disease, limbal stem cell deficiency, recurrent corneal erosion, superior limbic keratoconjunctivitis, persistent epithelial defects and NK.15,52-54
Corneal nerve regeneration has been documented in NK patients with the use of autologous serum dosed six to eight times a day for the first month, tapering to four times a day.55
The concentration (ranging from 20% to 100%), dosing and preparation methods for autologous serum vary widely in both the literature and clinical practice.53
Oxervate. This drug received FDA approval in August 2018 for the treatment of NK at any stage of the disease and is the first topical biologic agent approved for eyecare.40,41 Oxervate (cenegermin-bkbj ophthalmic solution 0.002%, 20 mcg/m, Dompe) is a recombinant form of human NGF that aims to target the underlying pathology of NK, rather than solely addressing its symptoms. The medication is structurally identical to the endogenous NGF protein found in human ocular tissue and works through binding of specific NGF receptors in the anterior segment of the eye to ensure the proper growth and development of neurons that, in turn, support corneal integrity.15,39 It is also the first such treatment to help prevent decreased vision or loss of vision caused by NK.39
Several studies supported the use of topical NGF in NK to restore corneal integrity and improve corneal sensitivity before Oxervate was approved.43,56-58 Two pivotal trials, REPARO and NGF0214, established the efficacy of Oxervate for stage 2 and 3 NK in the United States.39,59-61
Treatment for corneal nerve dysfunction can be challenging, with many options available. A thorough case history, proper diagnostic testing and prompt identification of the disease process can help minimize serious corneal complications and reduce the need for invasive surgery. Employing the latest in imaging technology, medical devices and topical treatments can also prevent progression to the later stages of disease. Whether clinicians choose to manage some or all of these conditions, they are equipped to identify corneal nerve dysfunction and ensure patients get the care they need to recover.
Dr. Sicks is an assistant professor at the Illinois College of Optometry and serves as a clinical attending in the Cornea Center for Clinical Excellence at the Illinois Eye Institute. She lectures and participates in research on specialty contact lenses.
1. Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15-47. 2. Walker HK. Cranial nerve V: the trigeminal nerve. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. Butterworths; 2011. 3. Müller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521-42. 4. Al-Aqaba MA, Fares U, Suleman H, et al. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94(6):784-9. 5. Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263-85. 6. Remington LA, Goodwin D. Clinical Anatomy of the Visual System E-Book. Philadelphia: Elsevier Health Sciences; 2011. 7. Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46(12):4485-8. 8. Srinivasan S, Lyall DAM. Neurotrophic keratopathy. In: Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. Philadelphia: Elsevier; 2013:205-211. 9. Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93(7):853-60. 10. Efron N. A proposed new measure of corneal sensitivity. Invest Ophthalmol Vis Sci. 2016;57(6):2420. 11. Minsky M. Memoir on inventing the confocal scanning microscope. Scanning. 1988;10(4):128-38. 12. Wilson T. Trends in confocal microscopy. Trends Neurosci. 1989;12(12):486-93. 13. Yorek MS, Davidson EP, Poolman P, et al. Corneal sensitivity to hyperosmolar eye drops: a novel behavioral assay to assess diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2016:57(6):2412-9. 14. Tubbs RS, Rizk E, Shoja MM, et al. Nerves and nerve injuries: Vol 1. Academic Press; 2015. 15. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107-31. 16. Pritchard N, Edwards K, Vagenas D, et al. Corneal sensitivity as an ophthalmic marker of diabetic neuropathy. Optom Vis Sci. 2010;87(12):1003-8. 17. Bower KS. Laser refractive surgery. Up To Date. www.uptodate.com/contents/laser-refractive-surgery. Accessed March 27, 2020. 18. Battat L, Macri A, Dursun S, et al. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108(7):1230-5. 19. Benitez-del-Castillo JM, del Rio T, Iradier T, et al. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20(1):30-2. 20. Salomão MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. J Cataract Refract Surg. 2010;36(6):1024-32. 21. De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141(3):438-45. 22. Salomão MQ, Ambrósio R, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35(10):1756-60. 23. Ambrósio R, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24(4):396-407. 24. Rao GN, John T, Ishida N, et al. Recovery of corneal sensitivity in grafts following penetrating keratoplasty. Ophthalmology. 1985;92(10):1408-11. 25. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-9. 26. Lambiase A, Sacchetti M, Mastropasqua A, et al. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmol. 2013;131(12):1547-53. 27. Mathers WD, Jester JV, Lemp MA. Return of human corneal sensitivity after penetrating keratoplasty. Arch Ophthalmol. 1988;106(2):210-1. 28. Tavakoli M, Kallinikos PA, Efron N, et al. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30(7):1895-7. 29. Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792-7. 30. Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye (Lond). 2006;20(7):837-9. 31. Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28(10):1261-7. 32. Kurbanyan K, Hoesl LM, Schrems WA, et al. Corneal nerve alterations in acute acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye (Lond). 2012;26(1):126-32. 33. Chern KC, Zegans ME. Ophthalmology Review Manual. Philadelphia: Lippincott Williams & Wilkins; 2000. 34. Versura P, Giannaccare G, Pellegrini M, et al. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37-45. 35. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930-6. 36. Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56 (2):1097-1107. 37. Chucair-Elliott AJ, Conrady C, Zheng M, et al. Microglia-induced il-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia. 2014;62(9):1418-34. 38. Labbé A, Liang O, Wang Z, et al. Corneal nerve structure and function in patients with non-Sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54(8):5144-50. 39. Yeu E. Nerve growth factor for NK. Ophthalmology Management. September 1, 2019. 40. United States Food & Drug Administration. FDA approves first drug for neurotrophic keratitis, a rare eye disease. August 22, 2018. 41. Kaufman MB. Pharmaceutical approval update. PT. 2018;43:659-61. 42. AAO 2019: Topical nerve growth factor provides novel treatment for neurotrophic keratitis. Practice Update. October 18, 2019. [Epub]. 43. Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye (Lond). 2003;17(8):989-95. 44. van Klink F, Alizadeh H, He Y, et al. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1993;34(6):1937-44. 45. Schrader S, Wedel T, Moll R, et al. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1345-9. 46. Grey F, Carley F, Biswas S, et al. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye. 2012;35(6):288-91. 47. Weyns M, Koppen C, Tassignon MJ. Scleral contact lenses as an alternative to tarsorrhaphy for the long-term management of combined exposure and neurotrophic keratopathy. Cornea. 2013;32(3):359-61. 48. Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84(8):826-33. 49. Khokhar S, Natung T, Sony P, et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24(6):654-60. 50. Mcgaughy AG, Gupta PK. In-office use of amniotic membrane. American Academy of Ophthalmology. EyeNet. February 2015. 51. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88(11):1467-74. 52. Shtein RM, Shen JF, Kuo AN, et al. Autologous serum-based eye drops for treatment of ocular surface disease. Ophthalmology. 2020;127(1):128-33. 53. García-Martín E, Pernía-López S, Romero Jiménez RM, et al. The use of autologous serum eye drops for the treatment of ocular surface disorders. Eur J Hosp Pharm. 2019;26(6):314-7. 54. Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens. 2015;41(3):133-40. 55. Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94(5):584-91. 56. Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347-51; discussion 1351-2. 57. Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338(17):1174-80. 58. Lambiase A, Mantelli F, Sacchetti M, et al. Clinical applications of NGF in ocular diseases. Arch Ital Biol. 2011;149(2):283-92. 59. Bonini S, Lambiase A, Rama P, et al. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1468-71. 60. Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332-43. 61. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14-26. 62. Bower KS, Weichel ED, Kim TJ. Overview of refractive surgery. Am Fam Physician. 2001;64(7):1183-90. 63. Lagali N, Germundsson J, Fagerholm P. The role of Bowman’s layer in corneal regeneration after phototherapeutic keratectomy: a prospective study using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2009;50(9):4192-8. |
