Understanding AMD Presentations and Prognoses
These clinical pearls can help you identify at-risk patients in your practice and care for them over the long haul.
By Jessica Haynes, OD
 |
Release Date: August 15, 2020
Expiration Date: August 15, 2023
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Identify the early signs of AMD.
- Discuss the clinical metrics that can document AMD-related changes.
- Identify dry AMD and the signs of conversion to wet AMD.
- Describe how disease progression will affect vision.
- Review the changing prognosis of AMD given new therapeutic options.
Target Audience: This activity is intended for optometrists engaged in the care of patients with AMD.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Jessica Haynes, OD, Charles Retina Institute
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 68806-PS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Hayes has nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
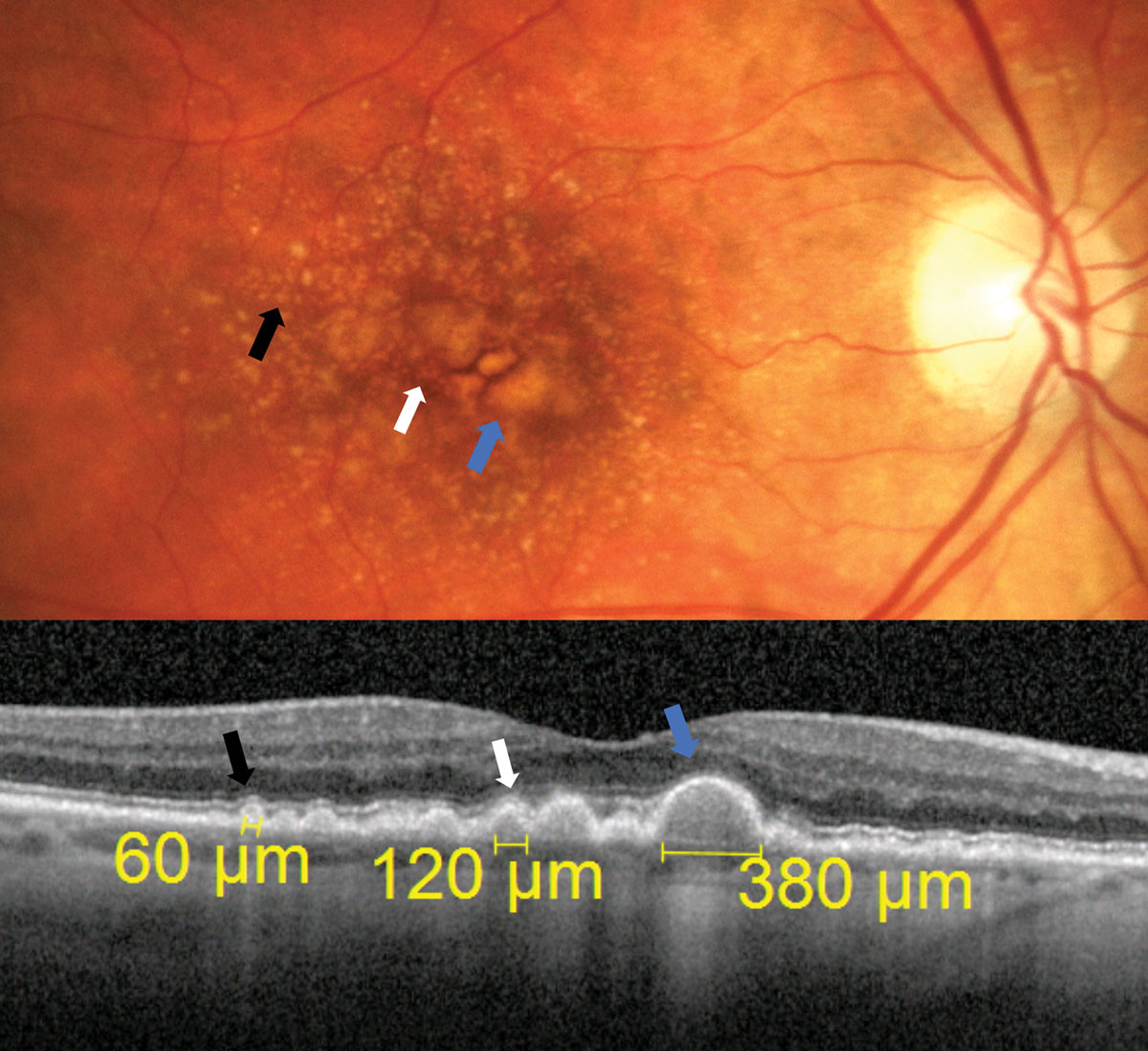 |
| Fig. 1. In these diagonal OCT cross section scans, the black arrow points to a subtle small sized druse, the white arrow points toward a medium sized drusen measuring 120µm and the blue arrow points toward a druse that is easily large sized measuring at 380µm. Click image to enlarge. |
To achieve best outcomes, patients with all levels of age-related macular degeneration (AMD) must be identified, properly educated and managed according to current evidence-based standards. Staging of the disease is crucial to determine risk of progression, recommended treatment/management and set an appropriate follow-up schedule. Clinical evaluation begins with the fundus exam, but additional diagnostic tools can help to further evaluate those with AMD, identify risk factors of progression and allow for earlier detection of all disease stages.
Funduscopic Evaluation
Even with significant advances in retinal imaging, the diagnosis of AMD is still most likely to stem from a thorough fundus exam. Findings associated with AMD may not always present in a dramatic fashion and can be masked by media opacity (e.g., cataract) or simply by a less-than-cooperative patient. This, along with other factors, may explain the results of a recent study that found 25% of AMD patients were misdiagnosed as having a normal macula. Of those misdiagnosed, 30% had signs consistent with intermediate AMD and would have benefited from nutritional supplementation.1
This type of misdiagnosis presents a huge missed opportunity for risk modification and appropriate patient education. Findings such as drusen, pigmentary alterations, geographic atrophy (GA) and macular hemorrhage can be signs of AMD and should be evaluated carefully, often with additional diagnostic tools.
Drusen are the hallmark finding of AMD. Their size is important in staging AMD and determining risk of progression. They are classified as small (<63µm), intermediate (between 63µm and 124µm) or large (≥125µm).2,3
Small drusen are subtle and require careful evaluation to visualize. A druse that is easily visible is likely medium to large sized. A major branch retinal vessel is about 125µm in width as it crosses the edge of the optic disc and is a good reference for grading drusen size funduscopically (Figure 1).4
AMD classification schemes used in major studies, such as the AREDS trials, are fairly involved and not easily incorporated into clinical practice.5 Fortunately, various authors have provided more clinically applicable summaries (Table 1).6
In general, patients with only a few small drusen are considered to have age-related changes, not AMD. Those with significant small drusen or any intermediate drusen are diagnosed with early AMD. Those with significant medium sized drusen, any large drusen or pigmentary alterations should be classified as intermediate AMD. Patients with the presence of either GA or choroidal neovascularization (CNV) have advanced stages of the condition.
Patients with CNV have exudative, or wet, AMD, and those without have non-exudative, or dry, AMD. Most notably, the AREDS trials found that those with intermediate-stage AMD benefited from nutritional supplementation.2
Patients with large drusen or any presence of pigmentary changes are at increased risk of developing advanced AMD. AREDS I Report No. 18 developed a point system that can help clinicians determine the five-year risk of progression to late-stage AMD based on the presence of these findings in each eye (Table 2).7
Table 1. AMD Classification & Management | ||
| AMD Stage | Fundus Findings | Management |
| Age-related Changes | Rare, small drusen | Reduce systemic and environmental risks. Monitor yearly. |
| Early AMD | Significant small drusen or any medium sized drusen | Reduce systemic and environmental risks. Recommend home monitoring with techniques such as Amsler grid. Monitor every six to 12 months, depending on risk. |
| Intermediate AMD | Significant medium sized drusen, any large drusen or pigmentary alterations | Reduce systemic and environmental risks. Discuss benefits of nutritional supplementation. Consider home monitoring device such as the ForeseeHome system. Monitor every four to six months, depending on risk. |
| Advanced AMD | Presence of GA or CNV | Reduce systemic and environmental risks. Discuss benefits of nutritional supplementation. Recommend home monitoring with techniques such as Amsler grid. Immediate referral for possible anti-VEGF injections for those with CNV. Monitor GA every six to 12 months, depending on risk. Consider low vision referral. |
Patients with these findings must be educated regarding the benefit of nutritional supplementation and their risk for conversion to advanced AMD. They should be monitored with increased frequency and heavily educated about the importance of home monitoring with the Amsler grid or ForeseeHome system (Notal Vision) for early detection of conversion to wet AMD.
Early identification of CNV remains of the utmost importance in preserving vision in those with AMD. Approximately 10% to 15% of AMD patients will go on to develop CNV, risking quickly progressive and severe central vision loss.8 The classic teaching of CNV appearance describes it as a “grey-green subretinal membrane,” but CNV can vary widely in its fundus appearance.
Certain features should raise a red flag for suspicion. These include the presence of macular hemorrhage, particularly subretinal hemorrhage, macular exudates and macular thickening or elevation that could indicate intra- or subretinal fluid as well as large pigment epithelial detachments (PEDs) (Figure 2).9
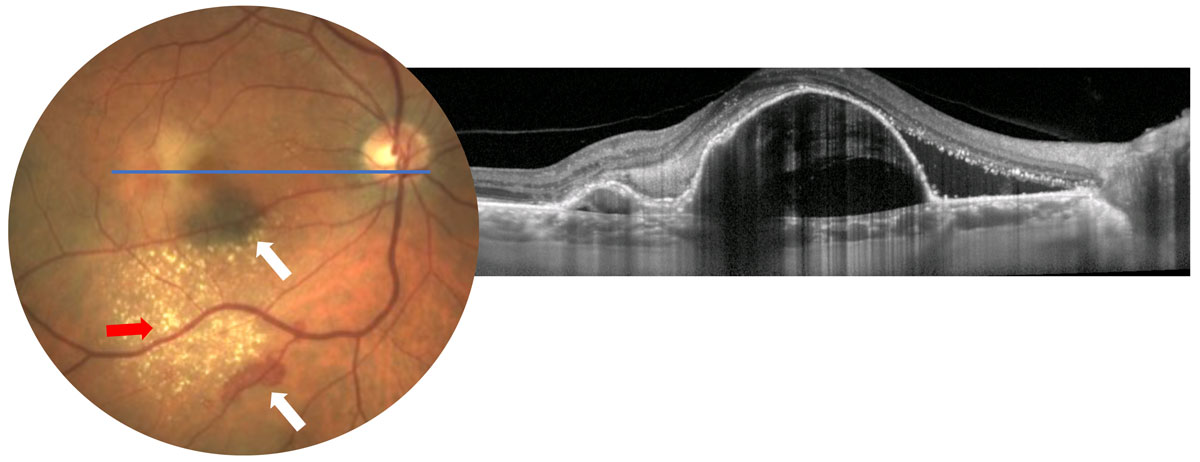 |
| Fig. 2. Possible CNV findings such as subretinal hemorrhage (white arrows), exudates (red arrow) or macular thickening (not readily visible in photo, OCT shows macular thickening from large PEDs) should raise a red flag for further testing. Click image to enlarge. |
In the setting of AMD, these findings with or without patient symptoms warrant further investigation with additional diagnostic tools.
Diagnostic Imaging
Many tried-and-true tools, as well as new technology, can help clinicians identify AMD:
Dye-based angiography. Intravenous fluorescein angiography (IVFA) was historically the go-to method for detection and classification of CNV. IVFA allows for classification of CNV as either classic or occult.
Classic CNV shows early, well-defined hyperfluorescence. Occult CNV shows patchy, ill-defined hyperfluorescence that appears later in the study (Figure 3).10 Due to these characteristics, researchers described classic CNV as a net that was located primarily between the retinal pigment epithelium (RPE) and the neurosensory retina, allowing easy visualization, and occult CNV as nets located primarily between the RPE and Bruch’s membrane where they could not be easily imaged.
These findings were verified with histological studies and optical coherence tomography (OCT) findings. Occult CNV is more commonly found in AMD than classic CNV.10,11
Dye-based angiography has largely been replaced in clinical practice with OCT for the diagnosis and management of CNV; however, this information remains relevant as it sheds light on the variable phenotypical presentation of CNV.12-14
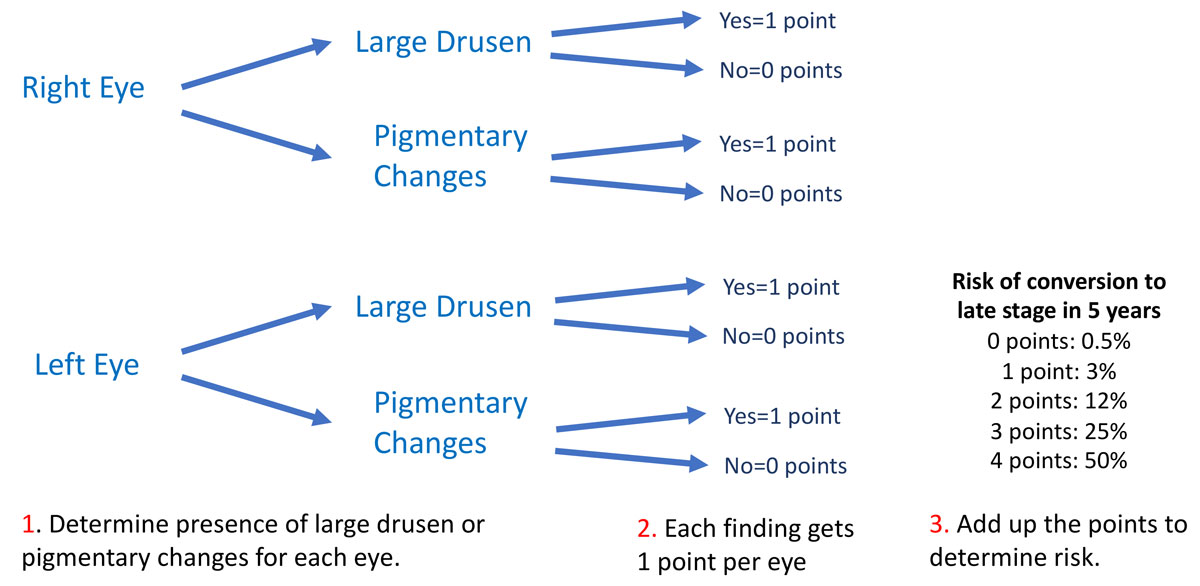 |
| Table 2. AMD Staging. Click image to enlarge. |
OCT. This has become the most widely used diagnostic tool in posterior segment disease, and its value in the evaluation and treatment of AMD is undeniable. OCT allows for visualization of retinal drusen, and many instruments also allow for measurement of drusen size. Typical drusen appear as moderately reflective deposits between the RPE and Bruch’s membrane.15
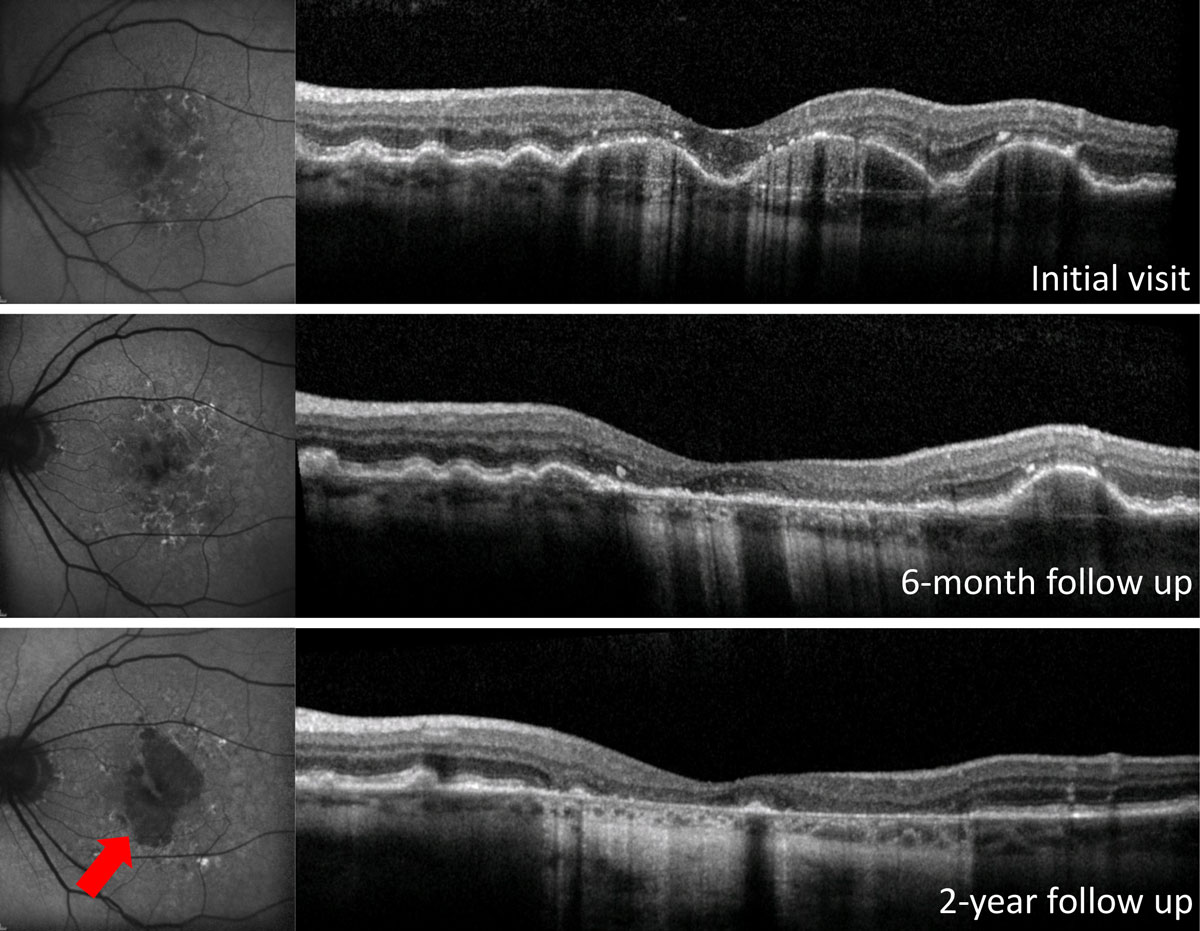
Drusen are transient, and over the course of the disease they can increase in size as well as resorb. A large regression of drusen can lead to new onset visual symptoms such as metamorphopsias, and it may be a precursor of advanced AMD, either formation of GA or CNV. Increased monitoring should be considered during periods of drusen regression. OCT can be used to monitor this clinical course (Figure 4).16-19
OCT can also help to identify a particular phenotype of drusen called reticular pseudodrusen (RPD), which presents as small, hyperreflective deposits that sit on top of the RPE and project upward toward the outer nuclear layer (ONL). They may project through the photoreceptor integrity line (Figure 5). This AMD phenotype is especially difficult to differentiate with fundus evaluation alone, and these drusen appear clinically as small- or medium-sized drusen.
Despite the size of RPD, patients with this presentation are at increased risk for both CNV and GA. Their risk is even higher than that of patients with large, soft drusen.20-26 Patients with RPD may also have poorer visual function than those without RPD.27-29 Identification of this finding warrants increased frequency of monitoring and patient education regarding higher risk of progression to advanced disease.
OCT imaging can also help clinicians identify GA, a sight-threatening complication of dry AMD. GA causes atrophy of the RPE and photoreceptors, leading to OCT findings of increased light penetrance into the choroid, loss of the photoreceptor integrity line and ONL atrophy.30
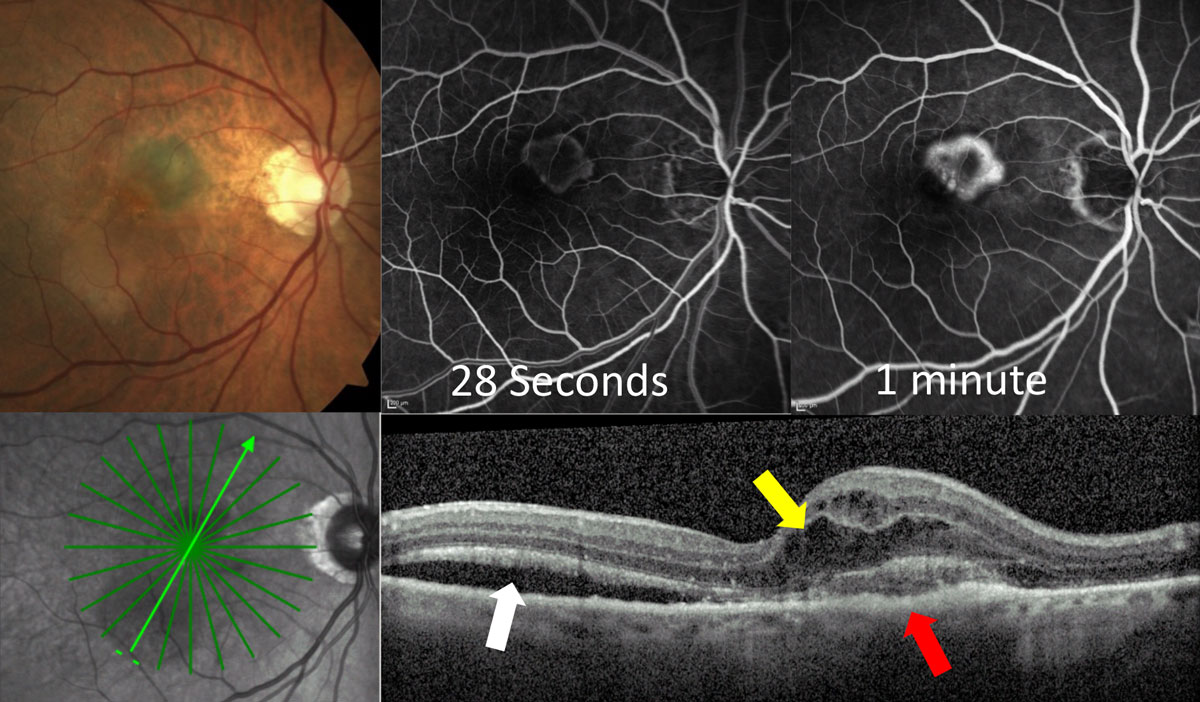 |
| Fig. 3a. This classic CNV shows early hyper-fluorescence at 28 seconds on IVFA with localized area of leakage at one minute. OCT shows the lesion primarily on top of the RPE (red arrow) with overlying intraretinal fluid (yellow arrow) and adjacent subretinal fluid (white arrow). Click image to enlarge. |
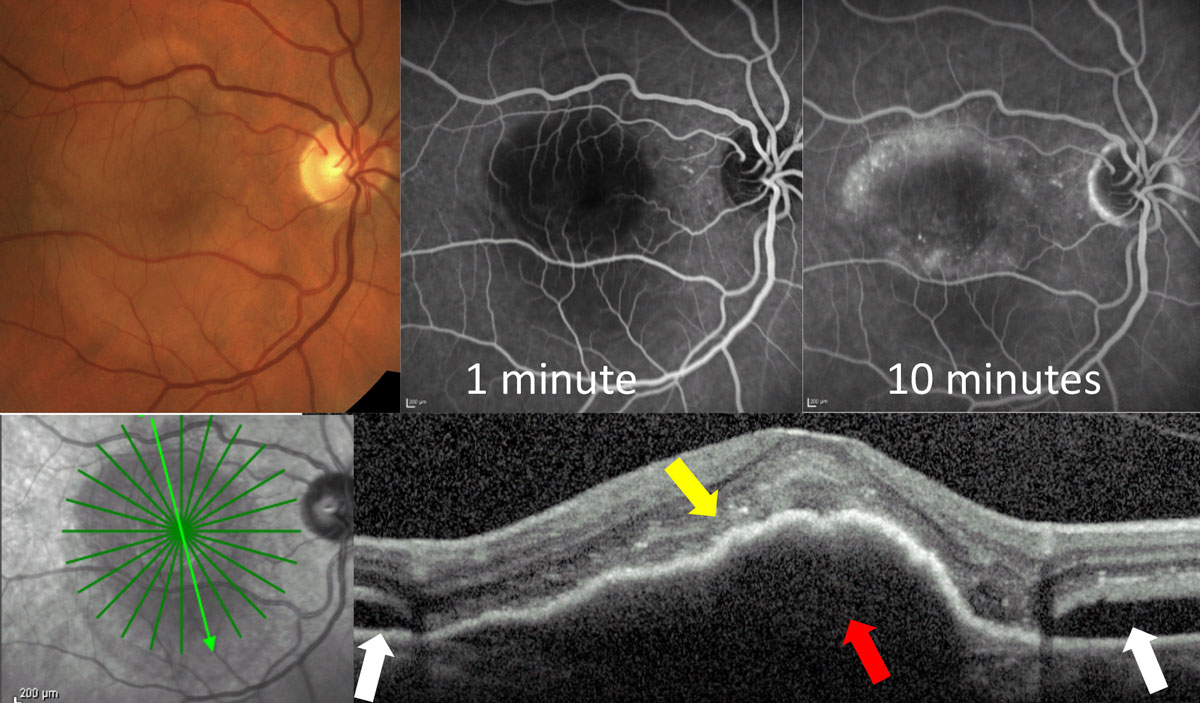 |
| Fig. 3b. This occult CNV shows no leakage early at one minute, and delayed patchy leakage in the later phases. OCT shows the lesion is a large PED (red arrow) with overlying subretinal hyper-reflective material (yellow arrow) and adjacent subretinal fluid (white arrows). Click image to enlarge. |
Perhaps the most notable benefit of OCT in AMD is identifying CNV and monitoring response to treatment. OCT imaging may detect CNV before clinical exam alone and should be considered for AMD patients with new visual symptoms or any clinical suspicion of CNV.
OCT findings present in CNV are variable and can include subretinal fluid, intraretinal fluid, PEDs and hyper-reflective subretinal material.12-14 Any sign of possible fluid on OCT should raise a red flag for concern of CNV in a patient with AMD.
Finally, it is important in patients with outer retinal disease to consider the status of the choroid, which can be visualized with OCT. Certain OCT imaging strategies, such as enhanced-depth imaging, allow for better visualization of the choroid and should be used when available.
In patients with AMD, the choroid tends to be relatively thin.31-33 Normal choroidal thickness varies due to factors such as age and refractive error but could be considered around 250µm. Choroidal thickness alone is not indicative of a singular disease, but trends can be observed that are useful in developing a more complete clinical picture.
Evaluating the choroid on OCT can help to differentiate AMD from certain masqueraders such as central serous retinopathy that tends to have thicker than average choroid (Figure 6).34
Fundus autofluorescence (FAF). This is an important diagnostic tool for patients with outer retinal disease. A light source of a particular wavelength is introduced into the eye and certain molecules, called fluorophores, will pick up the light source and autofluoresce. The most prevalent fluorophore in the retina is lipofuscin, primarily housed within the RPE. Disruptions to the RPE alter the autofluorescent signal. As RPE decreases and loses the ability for proper metabolism, increased lipofuscin concentration in the cells causes hyper-autofluorescence. In contrast, RPE cells that have atrophied show hypo-autofluorescence.35
FAF is particularly useful in identifying areas of GA and evaluating change over time. GA appears as very dense hypo-autofluorescence. GA with surrounding regions of hyper-autofluorescence is at an increased risk of growth.36
FAF is also helpful for patient education, allowing patients and family to understand that there are portions of the retina that see well, while other areas have atrophied, causing missing areas of vision.
This tool can also identify RPD, which has a very distinct FAF pattern. RPD present as small, hypo-autofluorescent circles in a reticular pattern on FAF. These hypo-autofluorescent circles sometimes have a hyper-autofluorescent core.35,37
In addition, FAF can be thought of as a general lipofuscin map or a map of the RPE health. Increased alterations to the normal autofluorescent pattern develop as there is worsening disease and decline in RPE function. FAF may demonstrate areas of disease that are not visible on fundus evaluation alone. Patients can present with similar funduscopic findings, but a more altered FAF likely indicates more advanced disease (Figure 7).35,37
 |
| Fig. 4. On FAF, at left, GA shows as prominent hypo-autofluorescence (red arrow). On OCT, at right, GA results in atrophy of the outer retinal layers, including loss of the photoreceptor integrity line and ONL. In addition, there is increased light penetrance into the choroid due to atrophy of the highly reflective RPE layer. Click image to enlarge. |
OCT-angiography (OCT-A). The use of OCT-A in AMD primarily pertains to its ability to detect vascular flow in the outer retina or avascular region of the neurosensory retina, which allows for detection of CNV. OCT-A can effectively identify both classic and occult CNV; in some cases, it can delineate the size of CNV better than IVFA. OCT-A is dyeless with fast image acquisition, allowing for more frequent testing with less risk.38,39
As a fairly new technology, physicians are still determining how to best interpret and use OCT-A test results. In regard to CNV detection, image artifacts can lead to both false positives and false negatives. Projection artifacts are an inherent problem with the technology. Essentially, blood flow in more superficial layers is projected into deeper slabs, making it seem that there is flow in these areas when there is not.
Current systems all have algorithms to decrease projection artifacts, but they are not eliminated entirely. If projection artifacts lead to the appearance of flow in the avascular region, this can give a false positive for detection of CNV when there is no true flow present.40-42 Other artifacts include movement artifacts, segmentation errors and shadowing. OCT-A images must be properly obtained and interpreted alongside additional clinical information to minimize diagnostic errors.40-43
With new technology often comes new clinical challenges, and OCT-A has identified a new subset of CNV. The terms non-exudative CNV or subclinical CNV are used to describe a CNV visible with OCT-A that has no evidence of fluid on the OCT B-scans or that does not appear to leak on IVFA.44-47
Patients with this finding have higher risk of developing exudation, but controversy still exists on the right time to refer or begin treatment. About 20% of subclinical CNV will develop exudation, and if the clinician elects to monitor these patients, they must be followed closely.45,46 Clinicians should consider a referral to a retina specialist (Figure 8).
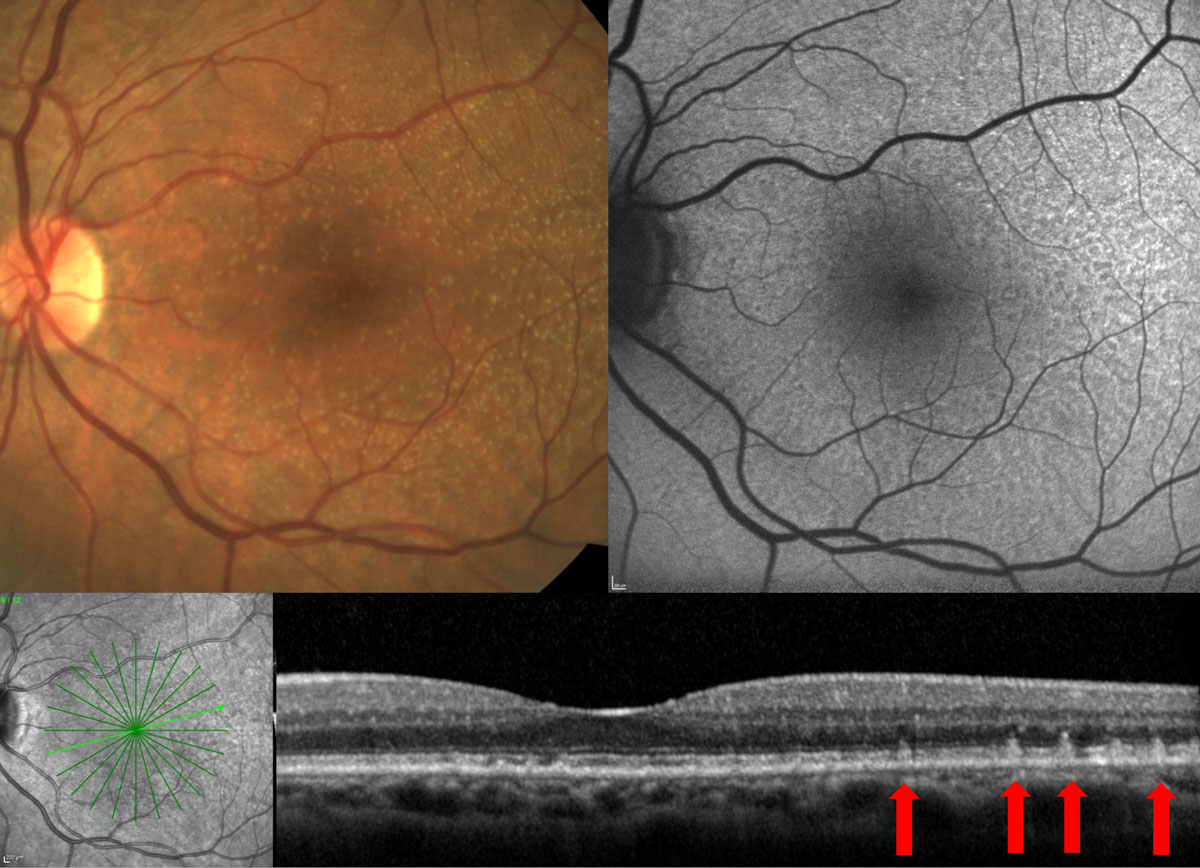 |
| Fig. 5. Fundus examination shows small- to intermediate-sized drusen; however, FAF and OCT show presence of RPD. Click image to enlarge. |
Visual Function Testing
While these tools each give unique insights into structural alterations of the retina that occur in AMD, they do not provide a measure of visual function. While multiple methods for testing visual function exist, dark adaptation has become a hot topic in AMD.
Recently, research has found that delayed dark adaptation precedes development of clinically visible macular degeneration by up to three years.48 AdaptDx (MacuLogix) can be used to measure dark adaptation in-office. This instrument was found to be 90% sensitive and 90% specific for identifying patients with AMD.49
This can be a useful tool for early identification of at-risk patients and early modification of environmental and systemic risk factors for progression. It can also aid patient education to demonstrate how visual function, and not just retinal structure, is affected.50 Dark adaptometry results must always be taken into context of the full clinical picture to arrive at an accurate diagnosis.
Progression and Vision
The physiological and functional milestones of AMD differ by type, as do our recommendations to patients:
Wet AMD. Among patients with AMD, 10% to 15% will develop CNV or wet AMD.8 This can lead to a sudden alteration in vision with symptoms such as blur, metamorphopsia and scotomas. Without treatment, patients are at a high risk for long-term, severe central vision loss. Early detection of wet AMD and prompt referral for treatment leads to better visual outcomes.51
Patients at significant risk for conversion to wet AMD, such as those with large drusen, pigmentary alterations or RPD, should be monitored more frequently. In addition, these at-risk patients should be educated extensively about monitoring monocular vision on a daily basis with tools such as the Amsler grid or ForeseeHome.
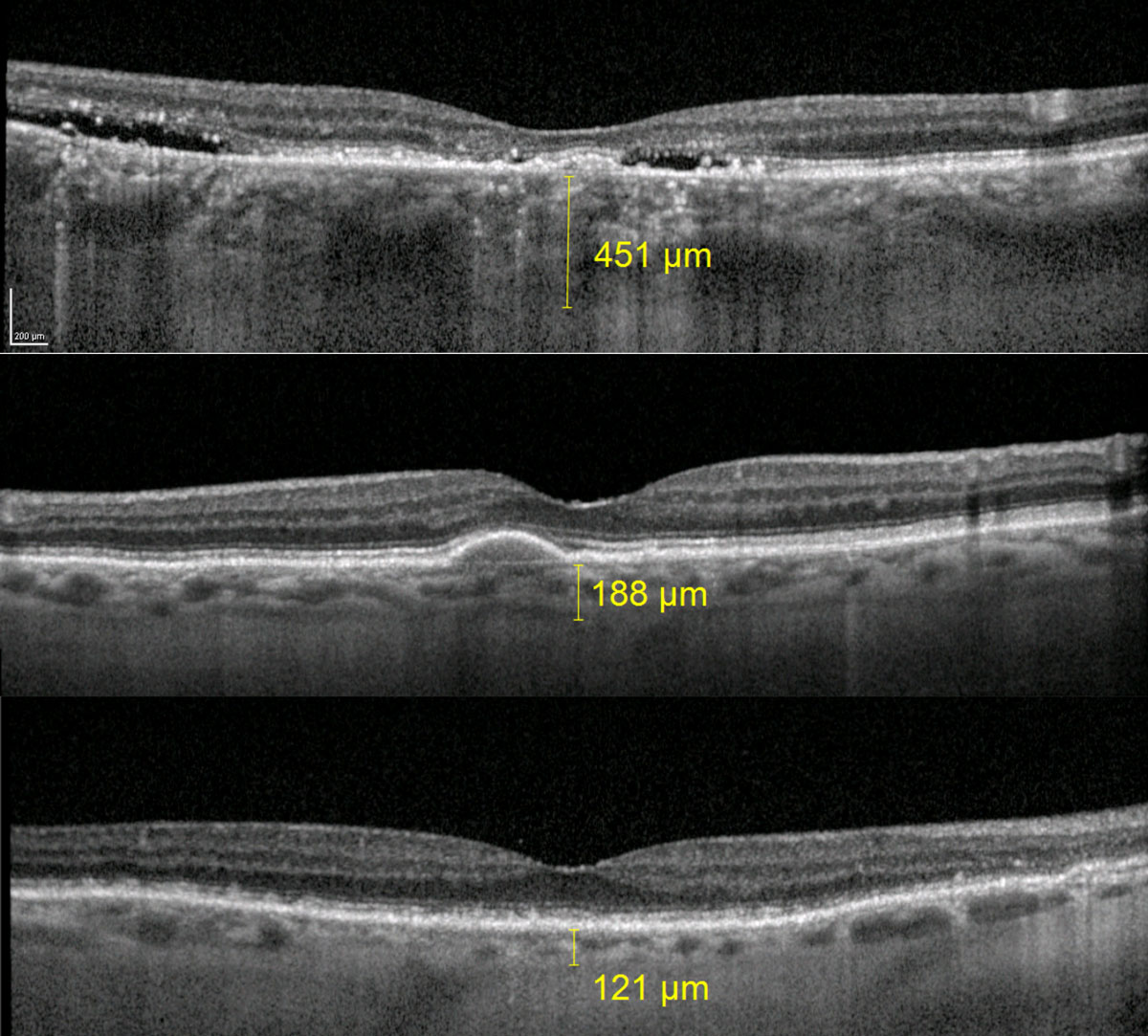 |
| Fig. 6. The top image is a patient with chronic central serous retinopathy and subfoveal choroidal thickness measuring 451µm. The middle image is a patient with large drusen from AMD and subfoveal choroidal thickness of 188µm. The bottom image is a patient with RPD and subfoveal choroidal thickness of 121µm. Click image to enlarge. |
The ForeseeHome system relies on hyperacuity to detect conversion to wet AMD. Patients can use the device at home to test their monocular vision daily. The results are monitored remotely through complex algorithms to detect alterations in the retinal structure.
Doctors receive an alert if the patient has been flagged for possible conversion. It is FDA approved for use in patients with intermediate, dry AMD.52 In a trial of 1,520 patients, 94% of those monitored with ForeseeHome who converted to wet AMD maintained 20/40 or better vision with treatment compared with only 62% of patients who converted while using standard monitoring techniques such as the Amsler grid.53
Dry AMD. Fortunately, 85% to 90% of patients with AMD never develop CNV.8 However, these patients may still suffer vision loss with variable severity and, unfortunately, treatment options are still severely limited. The most visually devastating consequence of dry AMD is the development of GA, which can lead to destruction of central vision and legal blindness.
Many patients with dry AMD maintain good visual acuity throughout their lifetime, but AMD affects vision in more ways than just central acuity. AMD is a degenerative, progressive disease of the choroid, RPE and photoreceptors. It is well established that patients with AMD suffer from decreased contrast sensitivity.54-56 While a patient may see 20/20 on a high-contrast vision chart, they may struggle to read the newspaper or see a restaurant menu in poor lighting.
AMD also decreases the retina’s ability to both light and dark adapt. For example, AMD patients need a longer time for their vision to adjust before leaving the exam room after a dilated fundus evaluation. This translates to many real-life challenges such as ambulating between rooms of differing illuminations or seeing to drive at night with oncoming headlights.
In addition, AMD may cause visual distortions, reduced color vision, scotomas and decreased reading speeds.57 These factors should be considered when prescribing optical correction or low vision devices for those with macular degeneration.
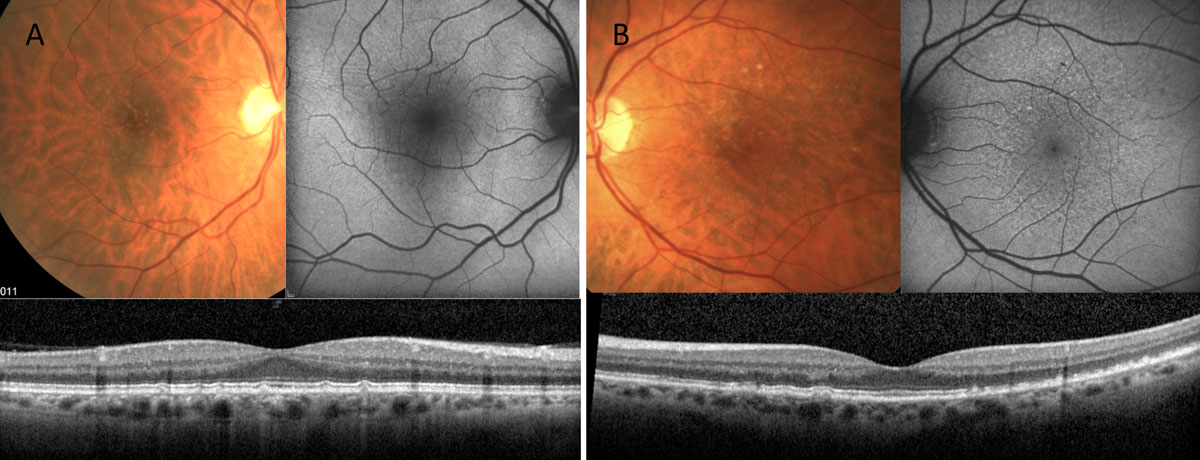 |
| Fig. 7. Both of these patients have small- to intermediate-sized retinal drusen. Patient A’s FAF shows fairly normal autofluorescent signal, while patient B has alteration to the FAF greater than the extent of disease seen clinically. Click image to enlarge. |
It is important to educate patients with both dry and wet AMD that while central vision can be severely affected, AMD does not lead to complete blindness. Many patients do not understand that there are varying levels of “blindness,” and instead assume that all blinding diseases lead to total darkness.
Treatment and Management
The approach to intervention is also dictated by the dry/wet distinction:
Wet AMD. Historically, wet AMD, although present in only 10% to 15% of AMD patients, was responsible for 90% of legal blindness from AMD. However, this is a statistic from the pre-anti-VEGF era. Intravitreal anti-VEGF injections can allow for stabilization or even improvement of vision in those that convert to wet AMD, but early detection is crucial to maintain good vision.
In the CATT trial, patients with better visual acuity and smaller CNV lesions at presentation were more likely to achieve better visual outcomes. Those with worse entering acuity gained more letters of acuity, but ultimately never achieved visual acuity levels as good as those with better entering vision.58
A retrospective study evaluated patients with entering acuity of 20/40 or better treated with anti-VEGF for CNV. At one year, 81% of eyes maintained 20/40 vision and 75% at two years.59 In many patients, this type of early detection may be the difference between maintaining driving level vision and an independent lifestyle or not. This is why patient education, including recommendations for at-home vision monitoring, is so important.
In clinical trials for the most recently approved anti-VEGF drug, Beovu (brolucizumab-dbll, Novartis), more than 50% of patients were maintained with 12-week dosing at the one-year mark, making it the longest acting drug on the market.60 However, the company recently updated Beovu’s label to include data on potential risks of retinal vasculitis and retinal vascular occlusion.61
No matter the drug used, anti-VEGF therapy for wet AMD carries a high treatment burden for patients and often their families or caregivers as well. Anti-VEGF is a treatment, not a cure, and requires repeat injections for years if not a lifetime. This requires countless office visits and procedures. Treatments themselves may also cause anxiety or discomfort for patients.
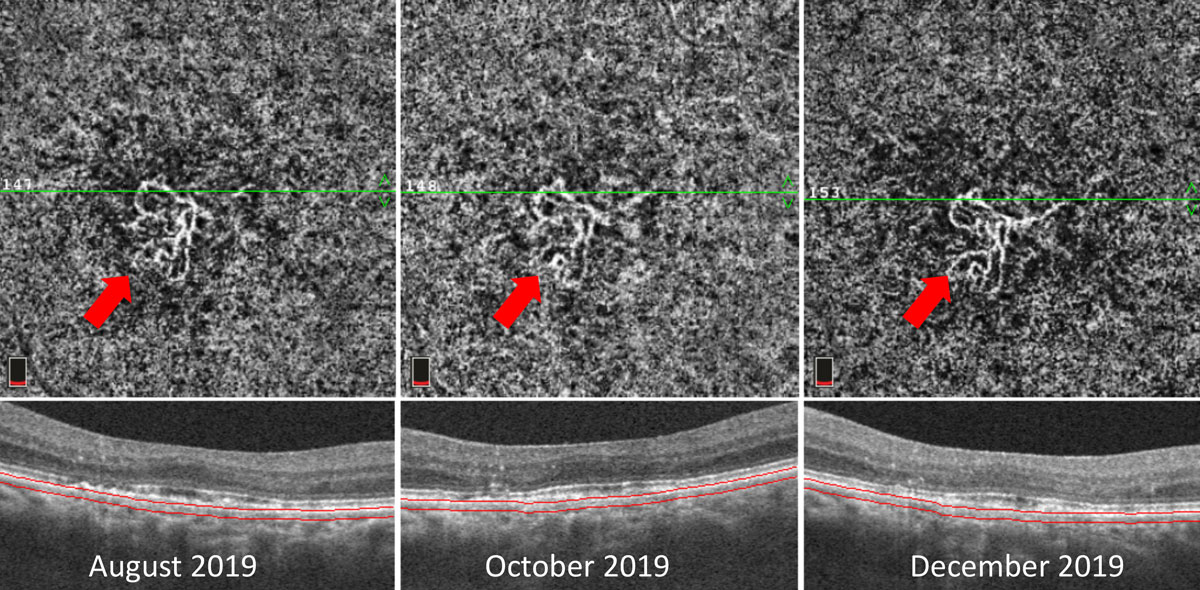 |
| Fig. 8. This patient with CNV located within the choriocapillaris slab of the OCT-A (red arrows) shows negligible overlying fluid on OCT cross section scans. The patient was followed over the course of four months with stable findings. This type of patient is at increased risk for the development of exudation and must be followed carefully or referred for consideration of treatment. Click image to enlarge. |
Dry AMD. Treatment for this condition remains limited. The AREDS I trial found that nutritional supplementation among patients with intermediate AMD resulted in a 25% risk reduction in the development of advanced stages of AMD.62 The AREDS II trial modified the original supplement formula based on its results, ultimately suggesting the following: 10mg lutein, 2mg zeaxanthin, 500mg vitamin C, 400 IU vitamin E, 80mg zinc, 2mg copper.62
A discussion of vitamin supplementation often devolves into controversy, as individual practitioners dispute which supplement is best to use and when to recommend it. Numerous studies report improvement of various metrics such as microperimetry, macular pigment optical density, contrast sensitivity and subjective visual improvement, among others, with the use of various vitamin supplements.63-70
However, there remains a lack of evidence that vitamin supplementation reduces the risk of developing advanced stages of disease in those with early AMD, and standard recommendations, such as those provided by the American Academy of Ophthalmology’s Preferred Practice Patterns, still suggest AREDS II type supplementation be reserved for those with intermediate stage AMD.71 Before recommending a supplement, clinicians must consider a patient’s systemic health, diet and medications, including other over-the-counter supplements they may already be taking.
| Genetic Testing Commercially available genetic testing is now an option for patients with AMD.74 The Macula Risk (ArcticDx) test aims to identify patients at increased risk of progression with use of zinc-based supplements.74 Controversy surrounding genetic testing still exists with no true consensus regarding the potential harm of vitamin supplementation.75-78 |
Another focus of dry AMD management is modifying environmental and systemic risk factors, such as diet, exercise and healthy sun protection habits. The number one modifiable risk for AMD is smoking.72,73 Systemic vascular disease, including hypertension, hyperlipidemia and diabetes, along with obesity all contribute to the disease.3
As treatment strategies for AMD evolve, our obligations for earlier disease detection grow larger. Now that we can better treat CNV, early detection is critical in the preservation of vision. Clinicians must continue to be diligent with fundus examinations to correctly diagnose AMD and identify high-risk patients. In addition, optometrists must understand the benefits of ancillary testing, such as OCT and FAF, and when these testing strategies are appropriate.
Dr. Haynes is an associate optometrist at the Charles Retina Institute and consulting faculty at Southern College of Optometry in Memphis, Tenn.
1. Neely DC, Bray KJ, Huisingh CE, et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-5. 2. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-36. 3. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313-30. 4. Goldenberg D, Shahar J, Loewenstein A, et al. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina. 2013;33(9):1888-94. 5. Age-Related Eye Disease Study Research Group. The age-related eye disease study (AREDS): Design implications AREDS report no. 1. Control Clin Trials. 1999;20(6):573-600. 6. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-51. 7. Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration. Arch Ophthalmol. 2005;123(11):1570-4. 8. American Academy of Ophthalmology. Age-related macular degeneration. https://www.aao.org/bcscsnippetdetail.aspx?id=9711f063-ed7b-452b-8708-c4dad0d893e8. 9. Kanski JJ, Bowling B. Clinical Ophthalmology: a Systematic Approach. 7th ed. Elsevier; 2011:611-28. 10. Tomi A, Marin I. Angiofluorographic aspects in age-related macular degeneration. J Med Life. 2014;7(Spec Iss 4):4-17. 11. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30(9):1333-49. 12. Regatieri CV, Branchini L, Duker JS. The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42(0):S56-66. 13. Parekh PK, Folk JC, Gupta P, et al. fluorescein angiography does not alter the initial clinical management of choroidal neovascularization in age-related macular degeneration. Ophthalmol Retin. 2018;2(7):659-66. 14. Gualino V, Tadayoni R, Cohen SY, et al. Optical coherence tomography, fluorescein angiography, and diagnosis of choroidal neovascularization in age-related macular degeneration. Retina. 2019;39(9):1664-71. 15. Jain N, Farsiu S, Khanifar AA, et al. Quantitative comparison of drusen segmented on SD-OCT versus drusen delineated on color fundus photographs. Invest Ophthalmol Vis Sci. 2010;51(10):4875-83. 16. Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age related macular degeneration. Retina. 2013;33(9):1800-8. 17. Yehoshua Z, Wang F, Rosenfeld PJ, et al. Natural history of drusen morphology in age related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12):2434-41. 18. Schlantz FG, Baumann B, Kundi M, et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. 2017;101:198-203. 19. Folgar FA, Yuan EL, Sevilla MB, et al. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology. 2016;123(1):39-50. 20. Joachim N, Mitchell P, Rochtchina E, et al. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014;121(4):917–25 21. Gil JQ, Marques JP, Hogg R, et al. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye (Lond) 2017;31(3):364-71. 22. Pumariega NM, Smith RT, Sohrab MA, et al. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619-25. 23. Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362-9. 24. Xu L, Blonska AM, Pumariega NM, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850-62. 25. Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121(6):1252-6. 26. Sawa M, Ueno C, Gomi F, et al. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina. 2014;34(4):761-7. 27. Ooto S, Ellabban AA, Ueda-Arakawa N, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2013;156(6):1184-91. 28. Ooto S, Suzuki M, Vongkulsiri S, et al. Multimodal visual function testing in eyes with nonexudative age-related macular degeneration. Retina. 2015;35(9):1726-34. 29. Querques G, Massamba N, Srour M, et al. Impact of reticular pseudodrusen on macular function. Retina. 2014;34(2):321-9. 30. Fleckenstein M, Issa PC, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137-44. 31. Corvi F, Souied EH, Capuano V, et al. Choroidal structure in eyes with drusen and reticular pseudodrusen determined by binarisation of optical coherence tomographic images. Br J Ophthalmol. 2017;101(3):348-52. 32. Lee JY, Lee DH, Lee JY, et al. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7812-8. 33. Farazdaghi M, Ebrahimi K. Role of the choroid in age-related macular degeneration: a current review. J Ophthalmic Vis Res. 2019;14(1):78-87. 34. Cheung CMG, Lee WK, Koizumi H, et al KB. Pachychoroid disease. Eye (Lond). 2019;33(1):14-33. 35. Ly A, Nivison-Smith L, Assaad N, et al. Fundus autofluorescence in age-related macular degeneration. Optom Vis Sci. 2017;94(2):246-59. 36. Choudhry N, Giani A, Miller JW. Fundus autofluorescence in geographic atrophy: a review. Semin Ophthalmol. 2010;25(5-6):206-13. 37. Bindewald A, Bird AC, Dandekar SS, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Opthalmol Vis Sci. 2005;46(9):3309-14. 38. Soomro T, Talks J. The use of optical coherence tomography angiography for detecting choroidal neovascularization, compared to standard multimodal imaging. Eye (Lond). 2018;32(4):661-72. 39. Nikolopoulou E, Lorusso M, Micelli Ferrari L, et al. Optical coherence tomography angiography versus dye angiography in age-related macular degeneration: sensitivity and specificity analysis. Biomed Res Int. 2018; https://doi.org/10.1155/2018/6724818. 40. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163-80. 41. Gao SS, Jia Y, Zhang M, et al. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT27-36. 42. Enders C, Lang GE, Dreyhaupt J, et al. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS One. 2019;14(1):e0210505. 43. Lauermann JL, Woetzel AK, Treder M, et al. Prevalences of segmentation errors and motion artifacts in OCT-angiography differ among retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1807-16. 44. Lane M, Ferrara D, Louzada RN, et al. Diagnosis and follow- up of nonexudative choroidal neovascularization with multiple optical coherence tomography angiography devices: A case report. Ophthalmic Surg Lasers Imaging Retin. 2016;47(8):778-81. 45. de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018:125(2):255-66. 46. Heiferman MJ, Fawzi AA. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS One. 2019;14(6):e0217805. 47. Bailey ST, Thaware O, Wang J, et al. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmol Retin. 2019;3(8):629-36. 48. Loshin DS, White J. Contrast sensitivity: the visual rehabilitation of the patient with macular degeneration. Arch Ophthalmol. 1984;102(9):1303-6. 49. Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427-31. 50. AdaptDx. https://www.maculogix.com/adaptdx. Accessed July 15, 2020. 51. Schwartz R, Loewenstein A. Early detection of age related macular degeneration: current status. Int J Retin Vitr. 2015;1(1):20. 52. ForeseeHome. www.foreseehome.com. Accessed JUly 15, 2020. 53. Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology. 2014;121(2):535-44. 54. Pondorfer SG, Wintergerst MWM, Gorgi Zadeh S, et al. Association of visual function measures with drusen volume in early stages of age-related macular degeneration. Invest Opthalmol Vis Sci. 2020;61(3):55. 55. Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106(1):55-7. 56. Faria BM, Duman F, Zheng CX, et al. Evaluating contrast sensitivity in age-related macular degeneration using a novel computer-based test, the Spaeth/Richman contrast sensitivity test. Retina. 2015;35(7):1465-73. 57. Chung STL. Reading in the presence of macular disease: a mini‐review. Ophthalmic Physiol Opt. 2020;40(2):171-86. 58. Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-9. 59. Rahimy E, Rayess N, Ho AC, et al. Treatment outcomes for neovascular age-related macular degeneration patients with initial vision better than 20/40 using a treat-and-extend regimen. Retina. 2016;36(5):875-80. 60. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. 61. Novartis provides update on use and safety of Beovu in patients with wet AMD. 2020. www.novartis.com/news/novartis-provides-update-use-and-safety-beovu-patients-wet-amd. Accessed July 15, 2020. 62. Kassoff A, Kassoff J, Buehler J, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436. 63. Corvi F, Souied EH, Falfoul Y, et al. Pilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementation. Br J Ophthalmol. 2017;101(6):770-3. 64. Falsini B, Piccardi M, Iarossi G, et al. Influence of short-term antioxidant supplementation on macular function in age-related maculopathy: a pilot study including electrophysiologic assessment. Ophthalmology. 2003;110(1):51-60. 65. Richer S, Devenport J, Lang JC. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78(5):213-9. 66. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75(4):216-29. 67. Beatty S, Chakravarthy U, Nolan JM, et al. Secondary outcomes in a clinical trial of Carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120(3):600-6. 68. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017. 69. Parravano M, Tedeschi M, Manca D, et al. Effects of Macuprev supplementation in age-related macular degeneration: a double-blind randomized morpho-functional study along 6 months of follow-up. Adv Ther. 2019;36(9):2493-2505. 70. Bartlett H, Eperjesi F. Age-related macular degeneration and nutritional supplementation: A review of randomised controlled trials. Ophthalmic Physiol Opt. 2003;23(5):383-99. 71. American Academy of Ophthalmology. Age-Related Macular Degeneration PPP 2019. www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp. Accessed July 15, 2020. 72. Myers CE, Klein BEK, Gangnon R, et al. Cigarette smoking and the natural history of age-related macular degeneration: the beaver dam eye study. Ophthalmology. 2014;121(10):1949-55. 73. Velilla S, García-Medina JJ, García-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147. 74. Arctic Medical Laboratories. https://arcticdx.com. Accessed July 15, 2020. 75. Chew EY, Klein ML, Clemons TE, et al. Genetic testing in persons with age-related macular degeneration and the use of the AREDS supplements: to test or not to test? Ophthalmology. 2015;122(1):212-5. 76. Awh CC, Lane A-M, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317-23. 77. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements. Ophthalmology. 2014;121(11):2173-80. 78. Assel MJ, Li F, Wang Y, et al. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2018;125(3):391-7. |
