 |
Trends in Infectious Keratitis
Here's what you need to know to better diagnose and treat this condition.
By William Skoog, OD, and Lindsay A. Sicks, OD
Release Date: February 15, 2022
Expiration Date: February 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss the current trends in infectious keratitis.
- Recognize the risk factors associated with this condition.
- Diagnose and treat corneal infections.
- Review how contact lens advancements have impacted infection rates.
Target Audience: This activity is intended for optometrists engaged in managing patients with infectious keratitis.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: William Skoog, OD, and Lindsay A. Sicks, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #123252 and course ID 76682-TD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Authors—Dr. Skoog has no financial interests to disclose. Dr. Sicks receives fees from Alcon. Managers and Editorial Staff—The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
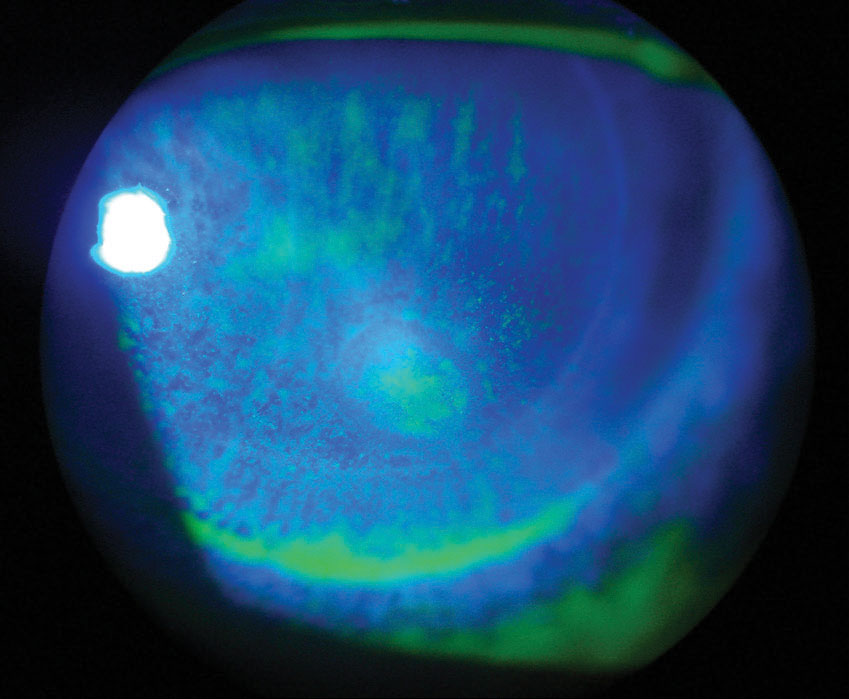 |
| Fig. 1. Microbial keratitis post-DSEK. Click image to enlarge. |
Infectious corneal ulcers are a leading cause of blindness worldwide, affecting approximately six million people globally.1,2 Most of this burden falls on the developing world, complicated by lack of access to proper hygiene and health care.
When these infectious corneal ulcers—also known collectively as infectious keratitis (IK)—are left untreated, the resulting corneal opacity can result in blindness. In 2019, the World Health Organization proposed that member states recognize IK as a neglected tropical disease, along with trachoma and onchocerciasis.3 The designation was an effort to increase awareness of, and funding toward, ending this corneal disease and its associated preventable blindness.3
IK can be caused by a variety of pathogens, including bacteria, viruses, fungi and parasites. The condition is commonly associated with contact lens wear; however, it can also occur secondary to trauma, corneal surgery and ocular surface disease (OSD). In this article, we will review some of the pathogens responsible for IK, along with additional risk factors for development of and best practices for managing this potentially blinding condition.
Bacterial Keratitis
Based on the difference in composition of the bacterial cell wall, bacteria can be categorized as either gram-positive or gram-negative. Gram-positive bacteria do not have an outer membrane; rather, they contain a thick outer cell wall. Gram-negative bacteria have cell walls that consist of a thin middle layer with an outer membrane containing lipopolysaccharide.4 The difference in composition of the bacterial cell wall can determine, in part, how the bacteria will affect the host immune system. It is also important to have a general understanding of the characteristics of both gram-positive and gram-negative pathogens to choose the most appropriate medication for a patient’s given disease.
Bacterial keratitis represents the most common type of IK globally, with polymicrobial infection accounting for up to 15% of all IK cases.2 In North America, bacterial keratitis accounts for 86% to 92% of all IK cases.5 A recent study found that bacteria was the causative organism in 95.1% of all IK cases.6 Gram-positive organisms were more commonly isolated than gram-negative organisms. Coagulase-negative Staphylococcus (CoNS) species, such as Staphylococcus epidermidis, are the most common gram-positive bacteria (25.7%), while Pseudomonas aeruginosa is the most common gram-negative bacteria (23.4%). CoNS are ocular commensal organisms found in the normal flora of the skin, eyelid and conjunctiva, whereas Pseudomonas aeruginosa is most commonly found in soil, water and vegetation.7
A closer look at the study’s population revealed that Pseudomonas aeruginosa was the most common pathogen in bacterial keratitis patients who wore contact lenses (57.9%).6 This data is similar to other studies done around the world.8-11 Researchers have also found similar results for the common causative organisms in bacterial keratitis occurring after corneal transplantation, with gram-positive bacteria (~40%) more commonly cultured than gram-negative (~20%) or fungal (~10%) species.12
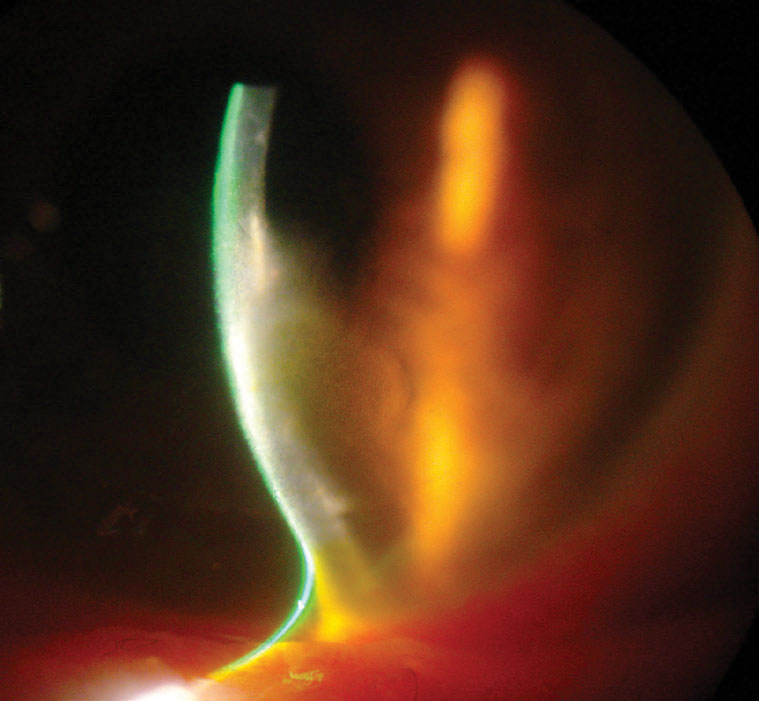 |
| Fig. 2. Corneal thinning and opacity in the same patient as Figure 1. Click image to enlarge. |
Fungal Keratitis
There are two broad categories of fungi—filamentous fungi and yeasts. The prevalence and epidemiological distribution of fungal keratitis are strongly associated with one’s geographical location and, thus, vary widely throughout the world.13 Overall, the worldwide incidence is 23.6 cases per 100,000 people, with the highest caseloads located in Asia and Africa.14 Filamentous fungi, such as Fusarium and Aspergillus, are the most common cause of fungal keratitis around the world and tend to be the predominant organisms in warmer, tropical climates.13 Yeasts, such as Candida species, tend to be found in more temperate climates, as well as in patients who are immunocompromised or have had chronic OSD or corneal surgery prior to infection.15
Though less common than bacterial keratitis, fungal keratitis can be more severe in nature. Corneal perforation is five- to six-times more likely to occur in fungal keratitis when compared with bacterial keratitis.16 In the developing world, fungal keratitis is strongly associated with ocular trauma from vegetable matter or other organic material. However, in the developed world, contact lens wear is the most common cause of fungal keratitis.13
Acanthamoeba Keratitis
A free-living parasite (amoeba, or single-celled organism) called Acanthamoeba is found in water and soil and is known to cause severe, sight-threatening infections.17 Most cases of Acanthamoeba keratitis (AK) have been observed in contact lens wearers, with an estimated prevalence of one to 33 per million contact lens wearers per year.18 AK most commonly occurs due to poor contact lens hygiene, such as topping off solutions or storing lenses in tap water.19 Patients typically present with symptoms that are out of proportion with signs (e.g., in extreme pain but with minimal corneal staining).
Targeted AK treatment is often delayed because the infection tends to be initially treated as a different type of keratitis (bacterial, fungal or viral). For example, in the early stages of infection, AK is often confused with herpes simplex viral keratitis if there is a dendritic pattern of epithelial staining. A more definitive diagnosis of AK can be made using in vivo confocal microscopy, though not every clinician has easy access to such an instrument.18
Acanthamoeba is especially adherent to the hydrophilic plastics used in contact lenses. For best outcomes, frequent replacement contact lens wearers using multipurpose solution should clean their case with clean fingers (digital rubbing), rinse it with multipurpose contact lens solution, wipe it with a tissue and leave it to air dry face down on a tissue with the caps off.20-22 The contact lenses should be rubbed, rinsed and stored using the recommended solution and following the manufacturer’s labeled instructions. Lenses should be replaced at the prescribed interval, and cases should be replaced regularly (every one to three months minimum).23
Patients using a hydrogen peroxide-based system with their soft contact lenses should rinse and store their lenses as directed by the solution manufacturer’s labeled instructions and take care not to apply hydrogen peroxide solution directly to the eye. All contact lens wearers should avoid rinsing or washing their case with tap water as this behavior increases the rate of contamination with gram-negative bacteria and Acanthamoeba strains.24,25 Daily disposable lens wearers should replace their lenses daily and avoid any re-use or storage.
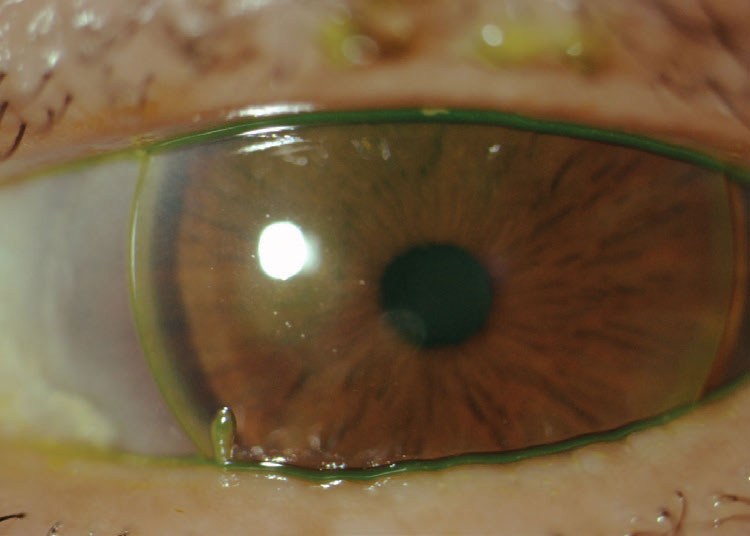 |
| Fig. 3. Patient with history of presumed microbial keratitis from continuous wear of spherical GP lenses showing an anterior stromal scar near the pupil. Click image to enlarge. |
Viral Keratitis
The most common viral ocular pathogens are herpes simplex virus (HSV) and varicella zoster virus (VZV). Other adenoviral pathogens, such as Epstein-Barr virus and cytomegalovirus, can also cause viral keratitis, though they are less commonly seen.26 Approximately 50% of the US population is seropositive for HSV-1, and the annual incidence of all types of new ocular HSV infections is 11.8 per 100,000 people in the United States.27,28 Typically, HSV-1 predominates in ocular infections.
Primary ocular HSV-1 infections tend to occur in children or young adults and typically present as a conjunctivitis, which can also involve the eyelids (blepharoconjunctivitis), marked by inflammatory vesicles and ulcers, which can include dendritic lesions on the corneal epithelium. More often, HSV-1 ocular infections occur in adults due to reactivation of the HSV which lays dormant in the trigeminal ganglion since the time of primary infection. Epithelial keratitis is the most common type of ocular HSV, comprising approximately 60% of cases.28 Stromal keratitis accounts for 20% to 48% of ocular HSV cases and may also present with epithelial lesions.26,28 Less common are the endothelial subtypes of HSV ocular infection and resultant cases of neurotrophic keratitis.28,29
VZV, or shingles infection, occurs during or following infection with the virus. The disease affects specific and discrete regions of the body, known as dermatomes. Approximately 50% to 72% of patients who develop VZV have ocular involvement, which then becomes known as herpes zoster ophthalmicus.26
Risk Factors to Consider
There are several common risk factors associated with IK, including contact lens wear, ocular trauma, OSD and a history of corneal surgery.
Contact lens wear. Despite changes in contact lens materials and uptake of more frequent replacement lenses, research has shown a relatively stable rate of microbial keratitis (MK) in contact lens wearers over the past 25 years, affecting roughly two to five per 10,000 of those who wear contact lenses on a regular basis.30-35
Research shows that a combination of blinking, tear flow and the regulatory elements of the corneal epithelium and the basal lamina in the healthy eye work together to form a formidable barrier and protect the vulnerable corneal stroma against microbial infection. It should be noted, however, that superficial trauma is not necessary for Pseudomonas aeruginosa to cross the corneal epithelium during lens wear and cause infection. Pseudomonas aeruginosa is the most common cause of contact lens-related infection.36 Silicone hydrogel lenses were developed, in part, to try and reduce infection rates in contact lens wearers. While the material innovation increased oxygen transmissibility to the cornea, uptake of silicone hydrogel lenses has not reduced rates of MK infection.36
In developed countries, contact lens wear is the number one risk factor for IK, in part due to modifiable risk factors such as hygiene. Poor hygiene practices including poor cleaning methods, sleeping in lenses, showering in lenses, poor hand hygiene, failure to replace lenses on time, “topping off” contact lens cleaning solution and lack of cleaning/replacing contact lens cases can all lead to an increased risk of IK.32,37-39 Infection rates are higher with planned replacement contact lenses when compared with daily disposable contact lenses. One major benefit of daily disposable lenses is that no contact lens solutions, cleaning or cases are necessary, as the lenses are simply discarded at the end of each wearing day.
Studies have shown that patients who wear daily disposable lenses are more compliant with on-time replacement compared with those who use extended wear contact lenses.40,41 Approximately 88% of daily disposable lens wearers in the United States reported following the manufacturer’s recommended replacement frequency, compared with 72% of monthly replacement lens wearers and 48% of two-week replacement lens wearers.41 Overwearing or sleeping in contact lenses increases one’s risk of developing IK.30,32,37,39 Several studies have also identified poor contact lens case hygiene as a major risk for the development of IK.32,37,38
Various types of bacteria and fungi can be found on contact lenses and in cases, including Fusarium, Candida, Pseudomonas and CoNS.42-44 One study examined different microbes found on daily disposables compared with planned replacement lenses and noted that after a normal period of wear for a daily disposable lens, the only microbe on the contact lens was CoNS, which is part of the normal ocular microbiota of the skin, eyelids and conjunctiva.43 The planned replacement lenses contained Pseudomonas species, CoNS and fungi (Candida and Aspergillus). Pseudomonas was the most commonly found bacteria on planned replacement lenses.
This finding is significant because CoNS has been shown to cause a milder form of keratitis, in contrast with Pseudomonas which can cause a more severe form of keratitis and progress quickly.43 Additionally, silicone hydrogel lenses show greater adhesion to Pseudomonas aeruginosa and Staphylococcus aureus when compared with hydrogel lenses.44
Both Fusarium and Candida species can form biofilms on contact lenses and cases, as well as case wells and caps. Approximately one-quarter of fungal keratitis cases are caused by contact lens wear, and the commonly isolated pathogen is Fusarium species. This rate accounts for the elevated incidence during times when ReNu with MoistureLoc (Bausch + Lomb) was commercially available.42,45 Topping off solution was implicated in the 2004 to 2006 Fusarium keratitis outbreak associated with ReNu with MoistureLoc contact lens solution.42 ReNu with MoistureLoc also demonstrated reduced efficacy after evaporation and instability at higher temperatures—two factors that could have contributed to the outbreak.46 During this outbreak, the rate of risk and incidence of Fusarium infection was three- to six-times higher than in previous years.45
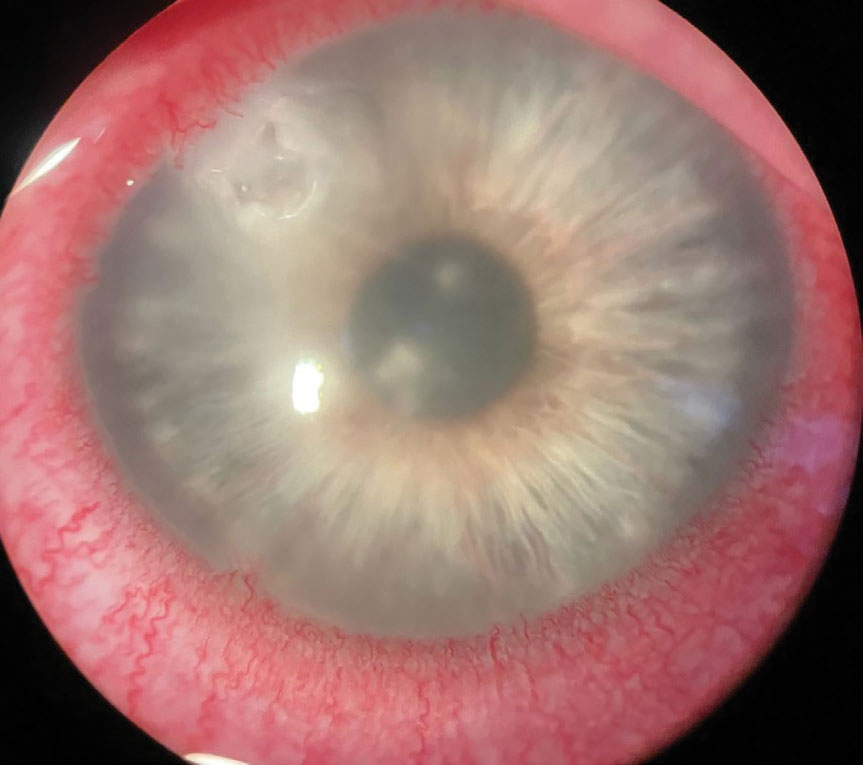 |
| Fig. 4. This case of Acanthamoeba keratitis was confirmed by confocal microscopy. Click image to enlarge. |
Corneal trauma. It is challenging to estimate the overall incidence of IK due to corneal trauma. In some studies, the language “corneal blindness” or “corneal trauma requiring topical antibiotic prophylaxis” is used to describe IK caused by trauma. The burden of corneal blindness from IK secondary to ocular trauma disproportionately falls on developing countries, where up to 90% of cases occur.47 The higher case numbers in developing countries could be due to the lack of access to, or availability of, prophylactic antibiotics.48 One study in Nepal showed that 96% of patients who started prophylactic topical antibiotics did not end up developing IK following corneal trauma, highlighting the importance of prompt treatment to prevent vision loss.49 In rural environments, IK cases are more commonly caused by trauma in association with contaminated water, vegetative matter such as soil and tree branches or wind-borne foreign bodies.48 Any trauma caused by vegetative matter increases the risk of fungal keratitis.
Fungal keratitis is also more common in working-aged males with occupations involving farming and manual labor and has a higher prevalence in Asian and African countries with agricultural communities. These trends highlight the importance of protective eyewear in high-risk environments to prevent not only direct infection, but also any corneal trauma that carries with it an elevated risk of infection and subsequent complications.
Corneal surgery. Due to its complex and invasive nature, keratoplasty carries a risk of IK. In the United States, the incidence of post-keratoplasty IK ranges from 0.02% to 4.1%.12,50 The majority of infections are caused by gram-positive organisms, the most common of which is Staphylococcus aureus.51 A single, large, retrospective study observed a higher incidence of MK following penetrating keratoplasty (PKP) than endothelial keratoplasty between 2007 and 2018 in the United States. This difference is attributed to long-term corticosteroid use, broken/loose sutures, recurrence of IK and OSD (including dry eye, neurotrophic keratitis and persistent epithelial defects). The study also noted that repeat grafts were more prone to infection than initial grafts.12,52 Other studies in the United Kingdom found similar results, with higher rates of IK following PKP compared with other corneal transplantation techniques.51 Over the past two decades, the incidence of post-keratoplasty bacterial keratitis has decreased; however, there has been a significant increase in post-keratoplasty fungal infection.53,54
IK following keratoplasty poses a diagnostic and therapeutic challenge for clinicians. Infection in eyes post-keratoplasty can lead to graft rejection, graft failure and endophthalmitis. Patients are usually on long-term topical corticosteroids to prevent graft rejection. However, during the active phase of an infection, steroids can worsen the clinical picture, especially in fungal keratitis. Yeasts tend to be the causative organism of fungal keratitis following corneal surgery, and recent studies have found a higher incidence of Candida infection following endothelial keratoplasty, highlighting the importance of proper diagnosis and treatment protocols in these complex cases.52
Keratorefractive surgeries, including laser in situ keratomileusis (LASIK), laser-assisted subepithelial keratectomy (LASEK) and photorefractive keratectomy (PRK), also carry a risk of IK. However, the incidence following refractive surgery is low at only about four per 10,000 eyes.55 IK incidence after LASIK was higher when compared with LASEK and PRK, and the most commonly cultured organisms were Staphylococcus bacterial species.55
OSD. This is a term that encompasses various forms of dry eye disease including keratoconjunctivitis sicca, Sjögren’s syndrome, blepharitis, cicatrizing conjunctivitis, Stevens-Johnson syndrome, post-refractive surgery dry eye, neurotrophic keratopathy, exposure keratopathy, bullous keratopathy, limbal stem cell deficiency and others.56,57 IK caused by OSD most commonly features gram-positive bacteria, such as CoNS, in culture results.56 Dry eye disease, as defined by the Tear Film and Ocular Surface Society’s Dry Eye Workshop II, features “a loss of tear film homeostasis with ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage and neurosensory abnormalities play etiological roles.” This loss of tear film homeostasis can lead to breakdown of the corneal epithelium, which is a vital part of the cornea’s defense mechanism.58 Further breakdown in corneal defenses, such as that seen in chronic inflammation, poses a continuous risk of OSD-related IK.2
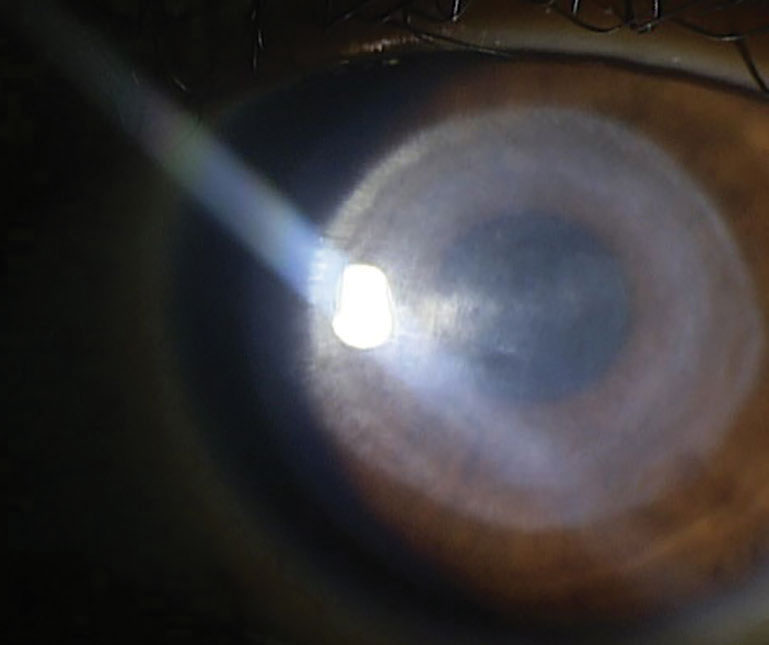 |
| Fig. 5. Central corneal scarring remaining after presumed Pseudomonas ulcer from extended wear of soft contact lens. Click image to enlarge. |
Diagnosis of IK
It is imperative that eyecare practitioners promptly recognize, diagnose and treat cases of IK. Patients will typically present with rapid-onset eye pain, conjunctival injection, photophobia and reduced visual acuity. The rate of symptom progression may depend on the virulence of the infectious agent. Clinical evaluation will typically reveal a corneal defect that stains with sodium fluorescein (NaFl) dye.
In cases of bacterial keratitis, one will usually see an infiltrate with an overlying epithelial defect. In HSV epithelial keratitis, there is often a typical dendritic corneal staining pattern—though sometimes the staining is non-specific in early stages. Some cases of IK have a more intense inflammatory response. For example, it is possible to see a marked anterior chamber reaction and associated hypopyon with some bacterial and fungal infections.
Following the American Academy of Ophthalmology guidelines for obtaining cultures and smears, a specimen should be obtained for laboratory evaluation if a presenting corneal infiltrate is central, large (>2mm) or has multiple sites of corneal infiltration.59 Additionally, a corneal culture should be obtained if there is significant stromal involvement or melting, the infection is chronic and unresponsive to broad-spectrum antibiotic therapy or there is any history of corneal surgery. Finally, if atypical clinical features are present suggestive of fungal, amoebic or mycobacterial keratitis (for example, a ring infiltrate or satellite lesion), an appropriate culture should be obtained. This culture may require special culture medium or plates, and a negative result does not necessarily rule out the suspected infectious agent.60
The clinical presentation of fungal keratitis tends to have less of an early inflammatory response than bacterial keratitis, though later in its course, conjunctival injection is fairly common. There is typically acute eye pain upon presentation, the severity of which can be out of proportion compared with the level of corneal inflammation detected.14 Fungal keratitis can manifest as gray-white, nonsuppurative infiltrates with irregular, feathery margins. Superficial lesions may be elevated with a dry, rough texture. Satellite lesions may also be present.60 Positive corneal cultures require several days or weeks for final identification, and sensitivity testing of any fungal isolate takes even longer, with questionable utility.61
AK can mimic HSV keratitis early in the disease process. Initially, the infection is localized to the epithelium and may present as diffuse epitheliopathy with coarse punctate features, subepithelial opacities or dendritic epithelial lesions. A ring infiltrate is present in about 50% of cases and is typically only seen in later stages of the disease. The patient classically presents with severe ocular pain that is out of proportion with the clinical signs. Features that favor a diagnosis of AK over HSV keratitis include the presence of epidemiologic risk factors, such as contact lens use or recent exposure to water (e.g., shower, hot tub or potentially contaminated freshwater). A good case history can help determine how likely the diagnosis is AK when the clinical picture is less clear.60
HSV keratitis can present with an epithelial or stromal lesion. Epithelial lesions will have the appearance of a dendrite and stain centrally with NaFl. Any heaped epithelium will stain with rose bengal or lissamine green, and the dendritic pattern will feature terminal end bulbs. These epithelial dendrites can progress to geographic ulcers, especially if a topical steroid is used without prophylactic antivirals. VZV keratitis presents as a pseudodendrite, which appears “stuck on’’ instead of ulcerated. Pseudodendrite lesions do not stain with NaFl and feature more blunted ends compared with the end bulbs seen in HSV keratitis.62
Treatment Options
The primary goal in treating IK is preserving the patient’s sight and corneal integrity. Any corneal perforation that develops can progress to endophthalmitis, so prompt identification and treatment are necessary. Generally, topical therapy should not be initiated until a corneal sample can be obtained for laboratory culture. Initial therapy usually consists of topical medications to combat the presumed infective agent, such as antibiotics, antifungals or antivirals. Adding a topical cycloplegic agent can reduce ocular discomfort and prevent any sequelae from intraocular inflammation if present.60
Initial treatment for bacterial keratitis consists of broad-spectrum topical antibiotics such as fluoroquinolones. Second-generation fluoroquinolones (ciprofloxacin, ofloxacin) have excellent gram-negative coverage and work well against Pseudomonas, but lack gram-positive activity. Newer, fourth-generation fluoroquinolones (moxifloxacin, gatifloxacin, besifloxacin) have similar gram-negative coverage, but feature improved gram-positive coverage. Combination therapy with separate antibiotics to obtain both gram-positive and gram-negative coverage is also acceptable. For more severe bacterial ulcers, consider culturing upon initial presentation in order to better target topical therapy and prevent antibiotic resistance. Fortified antibiotics are another option in severe cases where one has access to a compounding pharmacy.60
The initial recommended therapy for fungal keratitis includes topical natamycin 5% for filamentous fungi, particularly Fusarium species, or topical amphotericin B 0.15% for yeasts, such as Candida species.63 For more severe infections, or in fungal keratitis with scleral or intracameral extension, systemic antifungal agents may be needed.60
In cases of AK, early diagnosis is the single most important predictive factor for a positive outcome, as it is easier to treat trophozoites compared with cysts. Early in the diagnosis, epithelial debridement can be performed, followed by a three- to four-month course of topical therapy, the exact nature of which can vary. Topical therapies may include voriconazole, polyhexamethylene biguanide and chlorhexidine, among other antifungal agents, biguanides and antibiotics.
To prevent Acanthamoeba infection in contact lens wearers, avoiding exposure to water is key since few multipurpose solution preservatives are effective against this parasite. Hydrogen peroxide or povidone iodine (PI)-based solutions are preferred for any patient who may expose their contact lenses to water, as these are the only solution categories effective against Acanthamoeba.64 Unfortunately, PI solutions are not currently commercially available in the United States.
Primary ocular HSV infection is generally self-limiting; however, there are a few different approaches to treating HSV epithelial keratitis. Topical antiviral agents available in the United States are trifluridine 1% dosed nine times per day and ganciclovir 0.15% gel dosed initially at five times per day. Topical acyclovir ointment is currently only available abroad. While acyclovir ointment (Fera Pharmaceuticals) was FDA-approved in 2019 for acute herpetic keratitis, its commercial future is uncertain.65
Topical antiviral therapy is generally discontinued after a maximum of 10 days of treatment due to the high level of ocular surface toxicity. Common oral antivirals, such as acyclovir, valacyclovir and famciclovir, may also be prescribed to speed up the resolution of signs and symptoms in epithelial keratitis as they are not corneo-toxic. Once HSV progresses to stromal involvement, topical corticosteroids are required in combination with oral antivirals. Initial treatment for stromal disease is topical prednisolone acetate 1% dosed every two hours, along with prophylactic oral antivirals.62
Other potential treatment options for IK include corneal crosslinking (CXL), next-generation sequencing, novel antimicrobial agents (specifically to address drug resistance), photodynamic antimicrobial therapy and other adjuvant therapies that focus on modifying the immune response to treatment. CXL is a treatment typically reserved for keratoconus and corneal ectasia in which photochemically activated riboflavin promotes the formation of covalent bonds between corneal collagen strands. To date, there is little evidence to support CLX use to treat filamentous fungal keratitis; however, a stronger case can be made for its use in bacterial keratitis.66
Rose bengal photodynamic antimicrobial therapy has also been shown to be beneficial in severe, progressive IK cases, including those of fungal etiology.67,68 Next-generation sequencing techniques can improve diagnostic accuracy (and therefore help target treatment), especially in culture-negative IK cases. The sequencing can currently identify a wider variety of organisms, including atypical or anaerobic bacteria that are challenging to culture, but its role in targeting treatment is less clear.66
Further research in therapeutics may lead to novel antimicrobial agents which can be used to target various pathogens implicated in IK. Several case studies demonstrate the use of adjuvant therapies such as anti-collagenases, corticosteroids and systemic therapies, which can reduce corneal scarring and infection, thereby improving visual outcomes in IK.66,69,70
Conclusions
There are many risk factors associated with IK, though contact lenses have remained a constant risk, especially in cases of bacterial keratitis. Eyecare providers should also understand and consider additional risk factors for the development of IK such as corneal trauma, corneal surgery and OSD.
Bacterial keratitis case numbers have remained constant over the past 25 years, despite innovations in contact lens materials and the shift toward daily disposable lenses. With the advent of silicone hydrogel lenses in 1998, the eyecare industry was hopeful that the increased oxygen transmissibility would reduce the rate of MK, especially with extended wear. However, this change in material has had no appreciable impact on rates over time, which have held steady at two to five per 10,000 patients per year.30-35 Even with the rise in daily disposable contact lens use, which removes contact lens solutions and storage cases from the equation, the risk of severe infection is reduced but not completely eliminated.71
There is room for further improvement in reducing the incidence of IK, especially in contact lens wearers. Two potential innovations include the use of silver (or silver-salt) and cationic peptides. Silver iodide-infused galyfilcon A lenses performed similarly to normal galyfilcon A lenses, with no significant differences in comfort, acuity or ocular health.72 In vitro studies of silver-impregnated contact lens cases have also shown good efficacy against gram-negative bacteria. Contact lenses coated with melimine, a synthetic antimicrobial cationic peptide, show reduced bacterial adhesion with less gram-positive bacteria cultured from the eye.71
Keys to prevent IK include following best practices for safe contact lens wear, proper hand hygiene and the use of appropriate eye protection during any activity that carries a risk of ocular trauma. Despite the availability of appropriate treatments for many of the types of IK that exist, clinical outcomes are often poor and can vary based on geography and the availability of therapies. A better prognosis is associated with early diagnosis, access to appropriate treatment and use of prophylaxis in cases of ocular trauma.
The authors would like to thank Shivani Patel (ICO Class of 2022) for her assistance in obtaining Figure 4 from Dr. Anthony Verachtert, and Dr. Jennifer Harthan for her assistance in capturing Figure 5.
Dr. Skoog is a cornea and contact lens and ocular disease resident at the Illinois College of Optometry (ICO).
Dr. Sicks is an associate professor at ICO and a clinical attending in the Cornea Center for Clinical Excellence at the Illinois Eye Institute. She lectures and conducts research on specialty contact lenses.
1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-34. 2. Ting DSJ, Ho CS, Deshmukh R, et al. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye. 2021;35(4):1084-101. 3. Ung L, Acharya NR, Agarwal T, et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ. 2019;97(12):854-6. 4. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. 5. Tam ALC, Côté E, Saldanha M, et al. Bacterial keratitis in Toronto: a 16-year review of the microorganisms isolated and the resistance patterns observed. Cornea. 2017;36(12):1528-34. 6. Puig M, Weiss M, Salinas R, et al. Etiology and risk factors for infectious keratitis in South Texas. J Ophthalmic Vis Res. 2020;15(2):128-37. 7. Norina TJ, Raihan S, Bakiah S, et al. Microbial keratitis: aetiological diagnosis and clinical features in patients admitted to Hospital Universiti Sains Malaysia. Singapore Med J. 2008;49(1):67-71. 8. Tan SZ, Walkden A, Au L, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye. 2017;31(8):1229-36. 9. Mun Y, Kim MK, Oh JY. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS One. 2019;14(3):e0213103. 10. Tavassoli S, Nayar G, Darcy K, et al. An 11-year analysis of microbial keratitis in the South West of England using brain–heart infusion broth. Eye. 2019;33(10):1619-25. 11. Ting DSJ, Settle C, Morgan SJ, et al. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye. 2018;32(8):1416-7. 12. Dohse N, Wibbelsman TD, Rapuano SB, et al. Microbial keratitis and clinical outcomes following penetrating and endothelial keratoplasty. Acta Ophthalmol. 2020;98(7):e895-900. 13. Ahmadikia K, Aghaei Gharehbolagh S, Fallah B, et al. Distribution, prevalence, and causative agents of fungal keratitis: a systematic review and meta-analysis (1990 to 2020). Front Cell Infect Microbiol. 2021;11:698780. 14. Brown L, Leck AK, Gichangi M, et al. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21(3):e49-57. 15. Ritterband DC, Seedor JA, Shah MK, et al. Fungal keratitis at the new york eye and ear infirmary. Cornea. 2006;25(3):264-7. 16. Keay LJ, Gower EW, Iovieno A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001–2007: a multicenter study. Ophthalmology. 2011;118(5):920-6. 17. Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42(6):493-508. 18. Maycock NJR, Jayaswal R. Update on Acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35(5):713-20. 19. Brown AC, Ross J, Jones DB, et al. Risk factors for Acanthamoeba keratitis—a multistate case–control study, 2008–2011. Eye Contact Lens. 2018;44:S173. 20. Wu YTY, Willcox M, Zhu H, et al. Contact lens hygiene compliance and lens case contamination: a review. Cont Lens Anterior Eye. 2015;38(5):307-16. 21. Wu YT, Zhu H, Willcox M, et al. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010;51(12):6329-3. 22. Wu YT, Zhu H, Willcox M, et al. Impact of air-drying lens cases in various locations and positions. Optom Vis Sci. 2010;87(7):465-8. 23. Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. 24. Mutoh T, Ishikawa I, Matsumoto Y, et al. A retrospective study of nine cases of Acanthamoeba keratitis. Clin Ophthalmol. 2010;4:1189-92. 25. Tilia D, Lazon de la Jara P, Zhu H, et al. The effect of compliance on contact lens case contamination. Optom Vis Sci. 2014;91(3):262-71. 26. Koganti R, Yadavalli T, Naqvi RA, et al. Pathobiology and treatment of viral keratitis. Exp Eye Res. 2021;205:108483. 27. Young RC, Hodge DO, Liesegang TJ, et al. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128(9):1178-83. 28. Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17(1):40-9. 29. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-54. 30. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens–related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655-62. 31. Morgan PB, Efron N, Brennan NA, et al. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Invest Ophthalmol Vis Sci. 2005;46(9):3136-43. 32. Stapleton F. Contact lens-related corneal infection in Australia. Clin Exp Optom. 2020;103(4):408-17. 33. Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321(12):779-83. 34. Schein OD, McNally JJ, Katz J, et al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112(12):2172-9. 35. Cope JR, Collier SA, Schein OD, et al. Acanthamoeba keratitis among rigid gas permeable contact lens wearers in the United States, 2005 through 2011. Ophthalmology. 2016;123(7):1435-41. 36. Fleiszig SMJ, Kroken AR, Nieto V, et al. Contact lens-related corneal infection: Intrinsic resistance and its compromise. Prog Retin Eye Res. 2020;76:100804. 37. Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye. 2012;26(2):185-93. 38. Sauer A, Greth M, Letsch J, et al. Contact lenses and infectious keratitis: from a case-control study to a computation of the risk for wearers. Cornea. 2020;39(6):769-74. 39. Stellwagen A, MacGregor C, Kung R, et al. Personal hygiene risk factors for contact lens-related microbial keratitis. BMJ Open Ophthalmol. 2020;5(1):e000476. 40. Rueff EM, Wolfe J, Bailey MD. A study of contact lens compliance in a non-clinical setting. Cont Lens Anterior Eye. 2019;42(5):557-61. 41. Dumbleton K, Woods C, Jones L, et al. Patient and practitioner compliance with silicone hydrogel and daily disposable lens replacement in the United States. Eye Contact Lens. 2009;35(4):164-71. 42. Imamura Y, Chandra J, Mukherjee PK, et al. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52(1):171-82. 43. Hall B, McCanna D, Jones L. Identification of coagulase-negative staphylococci in daily disposable contact lens wearers. Lett Appl Microbiol. 2014;59(3):313-9. 44. Evans DJ, Fleiszig SMJ. Microbial keratitis: could contact lens material affect disease pathogenesis? Eye Contact Lens. 2013;39(1):73-8. 45. Zhang S, Ahearn DG, Noble-Wang JA, et al. Growth and survival of Fusarium solani-F. oxysporum complex on stressed multipurpose contact lens care solution films on plastic surfaces in situ and in vitro. Cornea. 2006;25(10):1210-6. 46. Bullock JD, Warwar RE, Elder BL, et al. Temperature instability of ReNu with MoistureLoc: a new theory to explain the worldwide Fusarium keratitis epidemic of 2004-2006. Trans Am Ophthalmol Soc. 2008;106:117-26. 47. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214-21. 48. Stapleton F. The epidemiology of infectious keratitis. Ocul Surf. 2021. 49. Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur Eye Study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85(4):388-92. 50. Edelstein SL, DeMatteo J, Stoeger CG, et al. Report of the Eye Bank Association of America Medical Review Subcommittee on Adverse Reactions Reported From 2007 to 2014. Cornea. 2016;35(7):917-26. 51. Griffin B, Walkden A, Okonkwo A, et al. Microbial keratitis in corneal transplants: a 12-year analysis. Clin Ophthalmol. 2020;14:3591-7. 52. Song A, Deshmukh R, Lin H, et al. Post-keratoplasty infectious keratitis: epidemiology, risk factors, management, and outcomes. Front Med. 2021;8:707242. 53. Sung MS, Choi W, You IC, et al. Factors affecting treatment outcome of graft infection following penetrating keratoplasty. Korean J Ophthalmol. 2015;29(5):301-8. 54. Lin IH, Chang YS, Tseng SH, et al. A comparative, retrospective, observational study of the clinical and microbiological profiles of post-penetrating keratoplasty keratitis. Sci Rep. 2016;6:32751. 55. Afsharpaiman S, Zare M, Yasemi M, et al. The prevalence of infectious keratitis after keratorefractive surgery: a systematic review and meta-analysis study. J Ophthalmol. 2020;2020:6329321. 56. Khoo P, Cabrera-Aguas M, Robaei D, et al. Microbial Keratitis and ocular surface disease: a 5-year study of the microbiology, risk factors and clinical outcomes in Sydney, Australia. Curr Eye Res. 2019;44(11):1195-202. 57. Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014;121(7):1398-405. 58. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-83. 59. Lin A, Rhee MK, Akpek EK, et al. American Academy of Ophthalmology preferred practice pattern cornea and external disease panel. Ophthalmology. 2019;126(1):P1-55. 60. Feder RS, et al. 2020-2021 Basic and Clinical Science Course, Section 8: External Disease and Cornea, Chapter 12: Infectious Diseases of the Cornea and External Eye: Bacterial, Fungal, and Parasitic Infections. American Academy of Ophthalmology, San Francisco. Published online 2021:317-51. 61. Tanure MA, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19(3):307-12. 62. Feder RS, et al. 2020-2021 Basic and Clinical Science Course, Section 8: External Disease and Cornea, Chapter 11: Infectious Diseases of the Cornea and External Eye: Viral Infections. American Academy of Ophthalmology, San Francisco. Published online 2021:281-316. 63. Sharma S, Das S, Virdi A, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99(9):1190-5. 64. Bradley CS, Sicks LA, Pucker AD. Common ophthalmic preservatives in soft contact lens care products: benefits, complications, and a comparison to non-preserved solutions. Clin Optom (Auckl). 2021;13:271-85. 65. Maulvi FA, Ranch KM, Desai AR, et al. Chapter 28 - Ophthalmic preparations. In: Adejare A, ed. Remington (Twenty-Third Edition). Academic Press; 2021:565-75. 66. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678-89. 67. Naranjo A, Arboleda A, Martinez JD, et al. Rose bengal photodynamic antimicrobial therapy for patients with progressive infectious keratitis: a pilot clinical study. Am J Ophthalmol. 2019;208:387-96. 68. Martinez JD, Naranjo A, Amescua G, et al. Human corneal changes after rose bengal photodynamic antimicrobial therapy for treatment of fungal keratitis. Cornea. 2018;37(10):e46-8. 69. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143-50. 70. Sharma B, Soni D, Mohan RR, et al. Corticosteroids in the management of infectious keratitis: a concise review. J Ocul Pharmacol Ther. 2021;37(8):452-63. 71. Szczotka-Flynn LB, Shovlin JP, Schnider CM, et al. American Academy of Optometry Microbial Keratitis Think Tank. Optom Vis Sci. 2021;98(3):182-98. 72. Lakkis C, Anastasopoulos F, Slater J, et al. Clinical performance of silver salt-infused silicone hydrogel contact lenses over six months of daily wear. Optom Vis Sci. 2011;88. |
