Time to Update Your Plaquenil Screening Protocols
The sooner you diagnose hydroxychloroquine-induced macular toxicity, the better, and new guidelines can help.
By Beth Norris, OD, and Sara Henney, OD, with Sara Weidmayer, OD
Release Date: August 2017
Expiration Date: August 15, 2020
Goal Statement: When caring for patients taking hydroxychloroquine, clinicians must be prepared to identify the earliest signs of macular toxicity, as the risk of progressive vision loss extends even after discontinuation. This article discusses the risks associated with hydroxychloroquine therapy and the updated American Academy of Ophthalmology guidelines designed to help clinicians better detect and monitor macular toxicity associated with long-term use.
Faculty/Editorial Board: Beth Norris, OD, Sara Henney, OD, and Sara Weidmayer, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 54366-PH. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Riviello and Michael Iannucci all have no relationships to disclose.
Plaquenil (hydroxychloroquine, Sanofi-Aventis) has been used as anti-rheumatic drug therapy for conditions such as systemic lupus erythematosus (SLE) and rheumatoid arthritis for years, despite its risk of irreversible macular toxicity. Historically, patients have been monitored using subjective measures such as visual field testing and objective findings during fundus examination, often leading to diagnosis of late-stage toxicity. However, it is crucial that clinicians identify the earliest signs of toxicity, as the risk of progressive vision loss extends even after discontinuation of Plaquenil. This is where new guidelines and advanced technology can help.
This article discusses the risks associated with Plaquenil therapy and the updated American Academy of Ophthalmology (AAO) guidelines designed to help clinicians better detect and monitor macular toxicity associated with long-term Plaquenil use. Several diagnostic tools are key to detecting macular toxicity as early as possible to ensure discontinuation of the drug before adverse sequelae threaten patients’ vision.
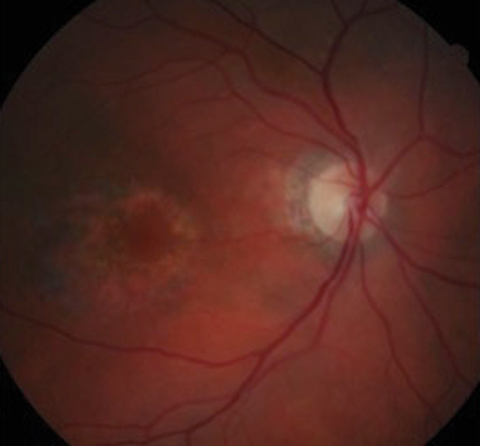 |
| Because fundus photography does not identify early findings, it is no longer a recommended screening tool. Photo: Marlon Demeritt, OD, MBA |
New Guidelines
In 2016, the AAO published updated ocular examination guidelines for screening patients on Plaquenil therapy (Table 1). Recommended testing still includes a comprehensive eye exam with dilated funduscopy, as well as 10-2 sita standard (ss) visual field (VF) testing using a white stimulus within the first year of use. A white stimulus is recommended over a red stimulus as it provides a pattern deviation plot and can distinguish regional loss from background sensitivity. The baseline examination should also include one objective test such as spectral-domain optical coherence tomography (SD-OCT), multifocal electroretinogram (mfERG), fundus autofluorescence (FAF) photos or a combination of the three. The guidelines no longer recommend supplemental screening tools such as fundus photography, Amsler grid, color vision, time-domain OCT, full field electroretinogram and electro-oculogram.1
The new guidelines recommend a maximum daily Plaquenil use of less than or equal to 5.0mg/kg/day of real weight, as opposed to ideal weight, as it correlates better with risk of toxicity. Researchers now know the drug is retained in adipose tissue, and short-statured or thin patients may be significantly overdosed if based on ideal weight. Risk is most accurately assessed on the basis of duration of use relative to daily dose by weight. However, wider test patterns (24-2 or 30-2) are needed for Asian patients in whom toxicity often manifests beyond the macula.2 Since these guidelines suggest additional testing, clinicians should educate patients of Asian descent on the need for more follow ups to perform the additional field testing.
The Risk
Although Plaquenil has a relatively safe systemic profile overall and Plaquenil macular toxicity is rare, clinicians must be cognizant of its potential for visual devastation. The 2016 AAO guidelines state the overall prevalence of toxicity in a five-year study population to be 7.5%—updated from the 2011 prevalence of 1% after five years of Plaquenil treatment.1,2
Each patient’s risk depends on daily dose and duration of use. At recommended doses (</= 5.0mg/kg/day of real body weight), the risk of toxicity is less than 1% up to five years and less than 2% up to 10 years.1,3However, this rises dramatically to almost 20% after 20 years of treatment.1-3 If the patient is taking the standard 400mg dosing per day, the calculated cumulative dose at seven years would be 1,022g.2
| Table 1. AAO 2016 Revised Screening Guidelines for Plaquenil Toxicity1 |
| Baseline exam—perform within one year of initiating treatment |
| Perform: |
| Visual acuity Dilated fundus exam 10-2ss visual field (white stimulus) 24-2ss or 30-2ss visual field (white stimulus) for Asian patients |
| One objective test: |
| SD-OCT mfERG FA |
| *Annual screening for toxicity will follow these same guidelines, and should begin five years after initiating Plaquenil therapy. |
Thus, patients who are taking higher dosages for longer durations are at an increased risk for developing both structural and functional changes.
In addition, many patients taking Plaquenil may concurrently be on methotrexate for the management of their autoimmune condition. Research shows these patients are at risk for optic neuropathy as well as Plaquenil toxicity.4 Thus, all Plaquenil screening protocols should be followed as well as periodic SD-OCT RNFL to monitor for optic neuropathy. These patients also should take folic acid to help to mitigate this risk.
Screening
The AAO guidelines recommend patients receive a baseline examination within the first year of Plaquenil use and an annual screening after five years of use, or earlier in higher risk patients (Table 2).1 Those at higher risk include patients taking a daily dose greater than 5.0mg/kg of real weight, more than five years of use, renal disease, concomitant tamoxifen use or macular disease.1 In a recent demographic study, researchers determined that age and risk of toxicity have no significant association, as elderly patients do not have less resistance to the toxic effects of Plaquenil.1 While the liver participates in the clearance of Plaquenil, no clear connection between liver disease and toxicity has been demonstrated.
The 2011 AAO guidelines recommended that concomitant macular disease be an exclusion criteria for Plaquenil use, as it masks the early signs of toxicity and renders screening less effective or impossible.2 Patients with underlying macular disease that interferes with the interpretation of screening tests should avoid taking Plaquenil.
As the risk increases two-fold from five to 10 years of use, high-risk patients, once they reach a cumulative dose of 1,000g, should be monitored more frequently for retinal toxicity.1,2
Case 1 Her medical history was significant for SLE, which was diagnosed 15 years earlier. For the last 14 years, she took 200mg Plaquenil BID. Her estimated cumulative dose was 1,080g. At her last visit one year ago, she was advised to discuss with her rheumatologist discontinuation of Plaquenil treatment secondary to findings of early toxicity. She is now taking methotrexate 250mg per week to replace Plaquenil. On examination, her best-corrected visual acuity measured 20/20 OU. Her pupils were equally round and reactive to light with no afferent defect. Confrontation visual fields were full to finger counting OU, and ocular motility testing was normal.
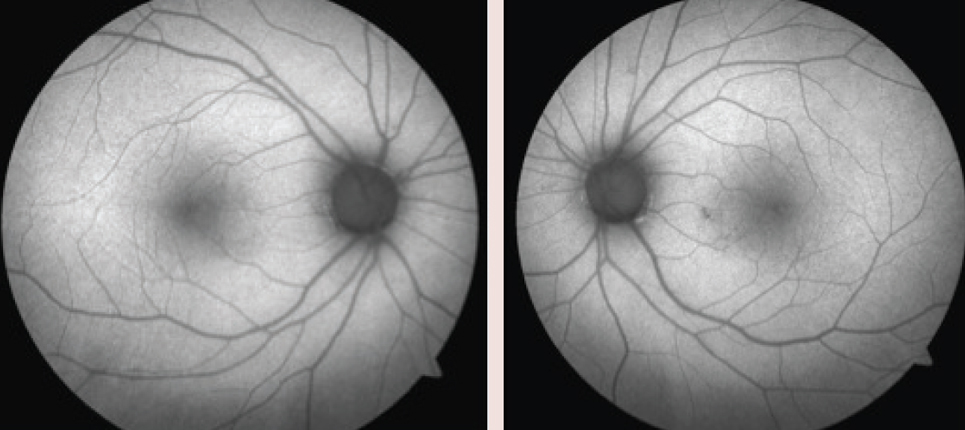
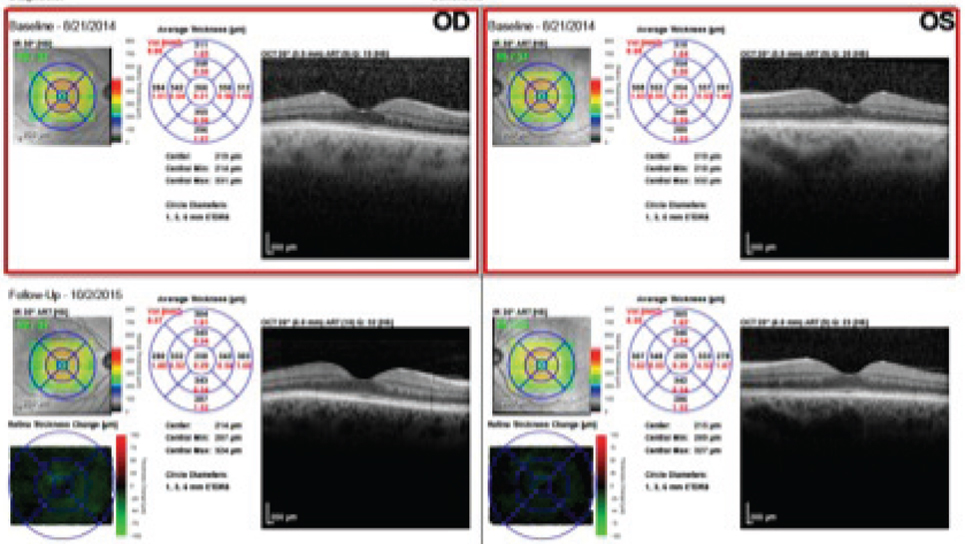
The anterior segment examination was remarkable for a reduced tear film and trace papillae OU. Her intraocular pressure (IOP) measured 15mm Hg OU. On dilated fundus examination, her optic nerves appeared healthy with average-sized cups with shallow slopes and good rim tissue and perfusion OU. The vessels were of normal caliber, and her peripheral retina was remarkable for a congenital hypertrophy of the retinal pigment epithelium (RPE) in the left eye. We noted changes in the RPE nasal paracentral to the macula in the left eye on the FAF photos (Figure 1). We also performed an SD-OCT of the macula (Figure 2).
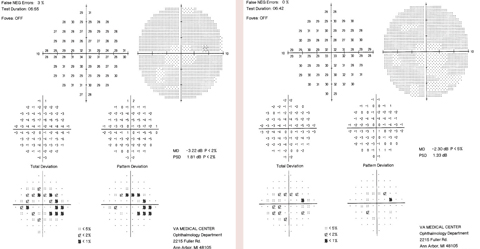
The patient has been followed with 10-2ss VF testing since the initial 2014 field, which showed a few scattered paracentral defects.The patient discontinued Plaquenil one week after the 2014 VF due to the signs of early toxicity. When the 10-2ss testing was repeated a year and a half later in 2016, it showed diffuse central/paracentral defects that had progressed since the patient had discontinued the medication (Figure 3).
The patient returned to the clinic three months after the 2016 VF testing, for an mfERG to better monitor the changes since discontinuing Plaquenil for more than a year (Figure 4). The mfERG showed evidence of macular depression in the left eye worse than in the right. No further treatment was recommended, considering long-term discontinuation of Plaquenil was already recommended one year prior.
We determined the patient had Plaquenil macular toxicity in the left eye, and the mfERG confirmed subclinical macular involvement in the right eye but did not have any signs of optic neuropathy in either eye. She is being followed closely every six months and is taking folic acid to mitigate this risk. |
Because of the decreased clearance of medication, patients with renal disease have an increased risk of circulating levels of Plaquenil. Clinicians must remain cautious with these patients, as they have unpredictably high blood-drug concentration levels and need close monitoring with more frequent follow-up visits.
Although the mechanism of action is unclear, patients undergoing concurrent tamoxifen treatment have a five-fold increased risk of toxicity.1 Concomitant use should be avoided, but if it is unavoidable or advised by the patient’s rheumatologist, these patients require more frequent follow-up visits for retinal toxicity.1
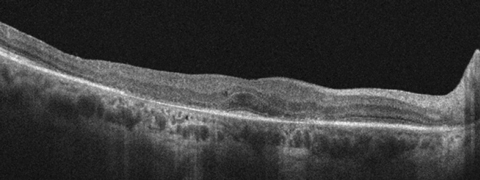 |
| This SD-OCT image of a patient on Plaquenil shows the classic flying saucer sign, which is a late-stage finding of toxicity. Photo: Marlon Demeritt, OD, MBA |
The Particulars of Dosing
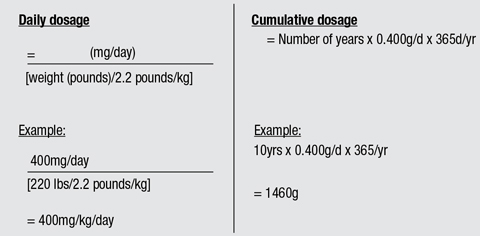
Calculating a patient’s dosing is first determined by commencement date of medication and daily dosing, based on their current body weight (in kilograms). According to the 2016 AAO guidelines, 5.0mg of Plaquenil per kg (of real weight) per day should be therapeutically effective for most patients (Table 3).1
What to Look For
The most common subjective patient symptoms in early stages of toxicity include difficulty reading, photophobia, metamorphopsia and reduced color vision.5,6 In later stages peripheral visual field loss and photopsia have been reported.5,6
The mechanism of action of Plaquenil is not well established, although research suggests Plaquenil alters the RPE lysosome PH, resulting in higher levels of lipofuscin, which has been associated with photoreceptor degeneration.6 In addition, Plaquenil binds to melanin of the RPE, which may serve to concentrate the agents and contribute to, or prolong, their toxic effects. Clinical findings in the anterior segment may reveal vortex keratopathy, which typically does not impact vision and is reversible after cessation of Plaquenil within six to eight weeks.5 The earliest clinical signs associated with Plaquenil toxicity are subtle changes at the macula that may be visualized as fine pigmentary mottling. In its late presentation, Plaquenil toxicity progresses to a symptomatic paracentral, and eventually central, scotoma with a characteristic bull’s eye maculopathy.6
Once there is evidence of toxicity, Plaquenil should be permanently discontinued, since its toxic effect is related to cumulative dose and could cause a permanent central scotoma, blindness or both. Because functional damage can occur prior to structural damage, early detection is key to reducing the patient’s risk of going blind.
| Table 2. Factors Increasing the Risk of Plaquenil Retinopathy | |
| Daily dosage | > 5.0mg/kg real weight |
| Duration of use | > 5 years, assuming no other risk factors |
| Renal disease | Subnormal glomerular filtration rate |
| Concomitant drugs | Tamoxifen use |
| Macular disease | May affect screening and susceptibility to Plaquenil |
Diagnostic Tools
When patients are taking Plaquenil long-term, clinicians can use SD-OCT and FAF to objectively monitor for possible retinal changes. However, evidence suggests electrophysiological changes occur prior to the structural changes identified on SD-OCT and FAF—indicating a need for earlier testing for high-risk patients.7 Both the 2011 and 2016 AAO guidelines suggest mfERG testing is an effective adjunct test.1,2 These specialized ancillary tests often make it easier to diagnose Plaquenil toxicity.
Automated threshold visual fields. The 10-2ss VF test is quite sensitive in reliable patients, but some tests are more reliable than others. The new guidelines recommend performing 10-2ss VF testing on non-Asian patients, and a wider test pattern (i.e., 24-2 or 30-2) in addition to 10-2 for Asian patients secondary to the risk of toxicity beginning beyond the central macula.
Proper field interpretation is key, as loss can be both parafoveal and extrafoveal. Also, any abnormality on the VF should be taken seriously. Uncertain VF changes should warrant retesting for both reliability and repeatability. If VF testing is inconclusive, the practitioner should consider evaluating with other objective tests, such as SD-OCT, FAF or mfERG.
| Case 2 He had no ocular or visual complaints. He had been monitored annually in our clinic for many years given his Plaquenil use, and there had been no evidence of Plaquenil-induced ocular toxicity to date. His baseline visual fields with white stimulus were normal.
On dilated fundus exam, findings in the right eye were normal, but the left eye’s macula was noted to have a subtle granular appearance to the superior parafovea. His macular OCT was normal with a full ellipsoid zone in both eyes. However, VF testing indicated decreased paracentral sensitivities in both eyes (Figure 1). Due to the concerning VF results, the patient was instructed to return as soon as possible for repeat, confirmatory testing including macular OCT, VF and photographs with autofluorescence. An mfERG at this point would have been ideal, but was not readily available. On follow up, his exam findings were stable and he had repeatable (though somewhat varied) VF defects. Photographs and autofluorescence imaging were unremarkable parafoveally. We contacted the patient’s rheumatologist with the recommendation to permanently stop Plaquenil use due to clear evidence of macular toxicity. Because visual loss from Plaquenil toxicity can continue to progress, even after the cessation of the medication, the patient has been followed at six-month intervals for monitoring. This case demonstrates why both structural and functional testing is important when evaluating for Plaquenil toxicity. Dr. Weidmayer practices at the VA Ann Arbor Healthcare System in Ann Arbor, Mich., and is a clinical instructor for the University of Michigan Department of Ophthalmology and Visual Sciences. |
SD-OCT. As early damage from Plaquenil occurs, SD-OCT shows localized thinning of the photoreceptor layers in the parafoveal region in non-Asians and near the arcades in many Asian eyes. An abnormality at the inner-outer segment junction on SD-OCT indicates structural change, which may appear before, concurrently or after functional change on VF testing. Researchers describe SD-OCT parafoveal defects associated with Plaquenil toxicity as a “flying saucer sign,” which is a late manifestation.2 SD-OCT may not be as sensitive as VF testing or mfERG, but when all ancillary testing is taken into consideration, this should allow for earlier detection of Plaquenil toxicity.1
SD-OCT is readily available in most offices, making it one of the most common testing modalities; however, one study suggested OCTs remained normal while VF 10-2 indicated parafoveal ring scotomas in 10% of toxic cases.8 At the same time, the researchers found no cases in which the opposite was true, when RNFL loss in the central five to 10 degrees was evident with OCT but showed no corresponding vision loss on the VF testing.8
FAF. With Plaquenil toxicity, research shows FAF has an increased signal with increased accumulation of lipofuscin and a decreased signal, indicative of RPE loss. Early Plaquenil toxicity can start as a fine pericentral ring of hyperautofluorescence, which can progress to pigment mottling and, subsequently, generalized hypoautofluorescence due to loss of pigmented epithelium. These changes can be more subtle than those found on SD-OCT.6
In one study, investigators looked at the ability of trained clinical observers to correctly indicate toxicity based on FAF or SD-OCT and found a 73.7% correlation of FAF abnormalities with toxicity and an 84% correlation of loss or disruption seen on the OCT with correlating toxicity.9
Table 3. Calculating Daily and Cumulative Dosage of Plaquenil
|
mfERG. This imaging technique can help to detect local variations in retinal function in multiple areas, by detecting the combined responses of photoreceptors, bipolar cells and Müller cells. It extracts hundreds of focal ERGs from a single electrical signal, creating a topographic map of retinal activity that allows clinicians to accurately assess a small area of the central retina.
Research suggests mfERGs can be sensitive enough to detect these variations in early Plaquenil toxicity.7 The retinal function is measured monocularly in response densities or amplitude per unit retinal area. These amplitudes are measured from the first negative wave to the first positive wave, in concentric rings from the center of the retina to the periphery. This format can detect central and pericentral depression, even when visual acuity and fundus findings are normal.2,10
Plaquenil toxicity shows a paracentral depression, or a decrease in amplitudes with prolonged implicit times associated with ring two (which correlates to the intersubfield of the OCT or the central five to 10 degrees).2,10
In one study, researchers used mfERG to confidently reassure patients of normal functioning retinas when a VF was abnormal; they also demonstrated that early Plaquenil electrophysiological changes may be reversible when the drug was discontinued early.11
| Sidelined Screening Tools Fundus photography. The clinician may choose to take fundus photos to establish a baseline and monitor for other maculopathies, but the AAO guidelines indicate photos are not sensitive enough to detect early Plaquenil changes.2,6 Amsler grid. Amsler grid monitoring is no longer recommended because most patients lack the understanding of how to perform the test accurately. In addition, patients are often unable to reliably discern the subtle changes associated with Plaquenil toxicity, unlike with wet AMD.2,6 Color vision. Clinicians may perform color vision testing, although it is recommended only at the initial baseline to rule out congenital color defects. The AAO guidelines also stress that color vision defects can be associated with many maculopathies and neuropathies, making it non-specific to Plaquenil toxicity.2,6 |
Other researchers suggest that a paracentral decrease in amplitudes and increase in latency on mfERG may be more specific to Plaquenil toxicity than VF testing and SD-OCT, allowing for the differentiation of maculopathy vs. vision loss from an optic nerve pathology. They conclude that mfERG testing may be a reliable objective test with good specificity for detecting and tracking Plaquenil toxicity.5
Yet another study found that the high false-positive rates of some studies suggest that cases detected by mfERG may be subclinical examples of retinopathy at risk of progressing to disease detected on other, morphology-based tests. Furthermore, the study suggests detectable electrophysiological changes precede morphological changes evident on other structural testing modalities such as the SD-OCT or FAF.7
One study suggested mfERG as the new standard testing modality for Plaquenil toxicity.9 mfERG diagnostic testing is both an objective measure and an evaluation of the retinal performance, possibly indicating its usefulness as a solitary screening tool.
Repeat Testing
Researchers have noted progressive damage after cessation of Plaquenil treatment up to three years after discontinuation.12 This suggests the need for repeating 10-2 VF testing after cessation of the medication. Early detection of Plaquenil toxicity is known to prevent visual acuity loss and serious progression after the therapy is stopped.13
The inconsistency of abnormalities seen on FAF, OCT and 10-2 VF testing and interpretation indicates the need for a better screening method. The untrained eye may not see changes in an atypical, high-risk patient, or may associate early changes to poor reliability during automated perimetry testing. Many clinicians may even dismiss early changes as inconsequential. But when these tests are taken in conjunction with one another, the specificity is quite high for detecting Plaquenil toxicity.
Regardless of the guidelines, clinicians should tailor the testing based on the individual patient. The bottom line for Plaquenil toxicity screening is simple: clinicians must recommend discontinuation of the medication at the earliest signs of toxicity, as this can result in permanent and progressive vision loss.
Drs. Norris and Henney are staff optometrists at the VA Health Care System in Orlando.
The authors would like to thank Saad Shaikh, MD, MBA, for his help collecting and interpreting the multifocal ERG testing.
1. Marmor MF, Kellner U, Lai T, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2016;123(6):1386-94. |