 |
The Final Cut: Surgical Correction of Presbyopia
Are we close to replicating accommodation without compromise?
Goal Statement:
This course reviews various surgical techniques and implantable medical devices used to correct presbyopia. Both currently approved and investigational products are discussed.
Faculty/Editorial Board:
Andrea Crabb, OD, and Ronald Krueger, MD
Credit Statement:
This course is COPE approved for 1 hour of CE credit. COPE ID is 43595-RS. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Drs. Crabb and Krueger have no financial disclosures relevant to this course.
Presbyopia, the age-related loss of the ability to actively focus on nearby objects, is a frustrating yet unavoidable condition of the eye. The exact mechanism is not fully understood, but changes in the ciliary body, zonules, lens capsule and lens itself are known to play a role. Symptoms usually begin around age 40 (“My arms are too short”) and progress until all accommodative ability is lost, usually in the mid-60s.
Everyone who lives long enough eventually develops presbyopia; this inevitability emphasizes the demand for good treatment options. Non-surgical management techniques such as glasses and contact lenses are popular and effective, but have shortcomings.
Some patients cannot adjust to multifocal spectacle lenses and must deal with the inconvenience of constantly switching between single vision distance and reading glasses. Others are bothered by the psychological stress of aging, which is confounded by the idea of needing bifocals, especially a cosmetically unattractive lined bifocal. Contact lenses can cause discomfort, exacerbate dry eye (a condition common in this older age group), and elevate the risk of potentially vision-threatening infections, such as corneal ulcers. Dexterity issues become more apparent with age, making it difficult for presbyopic patients to handle contact lenses. Also, patients with emmetropia or hyperopic presbyopia experience lens insertion difficulty from uncorrected near blur.
Advances in refractive surgery have led an ever-increasing segment of our patient population to seek spectacle and contact lens independence. Given its prevalence, effective surgical techniques to correct presbyopia are in high demand. The purpose of this article is to review current, new and investigational surgical solutions to presbyopia for the comanaging eye care professional.
Patient Management
Similar to the refractive surgeon, the comanaging optometrist faces the same challenges when dealing with the expectations of a premium IOL or refractive patient.
Often, patients seeking refractive surgery do not understand the challenges of treating presbyopia. Myopes have difficulty comprehending the sacrifice of nearsightedness at the expense of clear uncorrected distance vision. Hyperopes and astigmats often expect total independence from their full-time bifocal glasses, as relying on reading glasses defeats the purpose of refractive surgery. Additionally, the level of expense incurred can inflate visual outcome expectations. The optometrist and surgeon alike face the task of educating the patient and establishing realistic and achievable expectations. In general, a prudent approach is to under-promise and over-deliver.
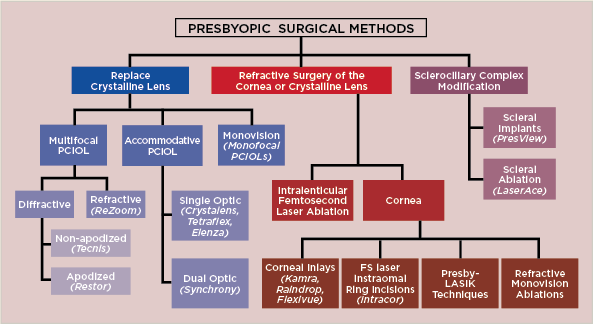 |
| Fig. 1. Current and experimental presbyopic surgical methods can be divided into three groups: replacement of the crystalline lens with a presbyopia-correcting intraocular lens (IOL), refractive surgery of the cornea or crystalline lens and modification of the sclerociliary complex. |
Good patient selection is critical to clinical success. Dissatisfied patients often complain of decreased contrast sensitivity and photic (halos/glare) symptoms, both of which are confounded by underlying corneal or retinal conditions. Therefore, dry eye, an irregular cornea, corneal astigmatism greater than 1D or any signs of macular pathology (e.g., epiretinal membrane, macular degeneration), would likely disqualify a patient from premium IOLs or refractive surgery.1 The ideal patient is motivated to be free from glasses and able to tolerate visual imperfection.
Current and experimental presbyopic surgical methods can be divided into three groups: replacement of the crystalline lens with a presbyopia-correcting intraocular lens (IOL), refractive surgery of the cornea or crystalline lens, and modification of the sclerociliary complex (Figure 1).1
Replacement of the Crystalline Lens
Presbyopia-correcting IOLs are categorized as multifocal or accommodative. Multifocal IOLs correct presbyopia using the principle of simultaneous vision, in which incoming light rays are divided into variable focal points, creating multiple coexisting retinal images. The patient primarily perceives only the focused image of interest (either distance or near) and ignores the blur from the unfocused image.2
• Multifocal IOLs are categorized as either refractive or diffractive, based on lens design. A third, emerging category uses a concept called rotational asymmetry (examples include the LentisMplus from Oculentis and the FineVision IOL from Liege).
Refractive IOLs create several focal points with concentric zones of varying optical power. Since the aperture of each zone varies, image quality depends on pupil size, which is driven by light and accommodation. The ReZoom (Abbott Medical Optics) is a refractive three-piece IOL with five refractive zones. Zones one (center), three and five focus distant images while two and four focus near images. The aspheric transition between zones provides intermediate vision while the near zones provide +3.5D of add power, which vertexes to +2.85D at the spectacle plane. The three distinct distance zones were designed to enhance distance vision in bright, intermediate and low light conditions. However, a small photopic pupil size may compromise near vision, given the lens’s center distance design.21
Diffractive IOLs, on the other hand, incorporate the principle of diffraction, in which light slows and changes direction when it encounters an obstacle. The surface of a diffractive IOL is covered with microscopic ridges, called diffractive zones. Incoming light is directed towards various focal points based on the diffractive zone’s ridge height and the light’s wavelength. The amount of light directed to a focal point is related to the step height as a proportion of wavelength. For example, at a step height of one wavelength, all light is directed to the near focal point, while at a step height less than one wavelength, light is directed to the distance focal point.2-4
Diffractive IOLs are subdivided into two designs. Apodized IOLs feature ridges that gradually decrease in height and spacing toward the lens periphery. As pupil size increases, more diffractive zones with smaller ridge heights are exposed, directing a larger proportion of light to the distant focal point, as described above. The AcrySof Restor (Alcon) is an IOL with a central apodized diffractive optic zone and refractive peripheral region. This design is believed to enhance distance vision in low light conditions, and offers an add power of either +4D or +3D, which vertexes to roughly +3.2D and +2.5D at the spectacle plane. A toric version of the Restor is currently available in Europe, but not yet approved in the United States.2,4
In contrast, the ridges on a nonapodized IOL are of uniform height and spacing from the center to the periphery, which directs an equal amount of light to the near and distance focal points independent of pupil size. Therefore, nonapodized lenses, such as the Tecnis multifocal IOL (Abbott Medical Optics), may enhance low contrast near acuity, but the optics can induce significant halos at night.2
Overall, both apodized and nonapodized diffractive multifocal IOLs have largely replaced refractive IOL designs due to reduced glare and halos and the improvement of other performance factors.5
• Accommodating IOLs fall into two categories: single-optic and dual-optic. Single-optic IOLs like the hinge-based Crystalens (Bausch + Lomb) alter image focal points through anterior movement of the IOL and changes in IOL architecture.6 In general comparison with multifocal IOLs, accommodating IOLs offer better contrast sensitivity and photic symptoms because incoming light is focused at a single focal point, similar to a monofocal IOL. The original Crystalens clinical trial, however, only reported about 1D of accommodation, so multifocal IOLs still provide stronger near power than current accommodating IOLs.
Various pseudo-accommodative factors can impact the accommodating IOL outcome—for example, modified monovision and pupil-dependent depth of focus. Capsular fibrosis can also interfere with the outcome by further reducing accommodation, inducing IOL tilt and possible asymmetric folding at the haptic-optic junction. This phenomenon is known as the “Z syndrome” because of the shape of the distorted IOL.7
To enhance the range of accommodation, dual-optic IOL systems use two lenses, an anterior high plus lens coupled to a posterior minus lens. As the distance between the two lenses changes, the effective optical power of the system is altered. For example, the Synchrony IOL (Visiogen/AMO) consists of a +32D front optic connected by spring haptics to a posterior negative optic of variable power. The lens is not currently approved by the FDA and the manufacturer appears to have ceased development, but trials show an accommodative range of 3.22 + 0.88D.8 Initial studies of the Synchrony system have also shown reduced posterior capsular opacification attributed to the non-collapse of the capsular bag with the dual optic design.9 However, a larger incision is needed for placement, which can induce postoperative astigmatism.10
Currently, the only FDA-approved accommodative IOL is the Crystalens and its toric version (Trulign), but other accommodating IOL systems are in clinical trials or development. The Tetraflex (Lenstec), a flexible non-hinged single-optic accommodating IOL, is one such example currently pending FDA approval. This IOL has closed-loop haptics that are angled anteriorly 10°, causing the optic to flex and change curvature with accommodation. A study done on the comparison of functional reading ability provided by the Tetraflex and the Crystalens noted a higher proportion of patients could read 80 words per minute or more at print sizes as small as 20/25 with the Tetraflex.10
Another investigational accommodating IOL that changes curvature with accommodation is the FluidVision Lens (PowerVision), an acrylic implant filled with silicone oil. As the ciliary muscle contracts and relaxes, energy is transferred to the lens zonules and lens capsule. This energy squeezes fluid from the haptics into the optic, increasing its anterior curvature. The Dynacurve (NuLens) also uses a fluid reservoir that can be reshaped to adjust the accommodative power of the lens.
Also under development is the Sapphire AutoFocal Elenza (PixelOptics), which changes power when accommodation is sensed, using a process its developer calls “electroactive optics.” A microscopic battery in this electronic implant stimulates internal liquid crystals when pupil size decreases; however, unlike the FluidVision lens, the Elenza implant is independent of the ciliary muscle and capsular bag biomechanics.
Similar to presbyopia, the development of cataracts is also common in those who live long enough. As cataract extraction with the insertion of a presbyopia-correcting IOL kills two birds with one stone, the ideal multifocal or accommodating IOL candidate is a patient requiring cataract surgery with no underlying ocular pathology. A refractive lens exchange is the removal of the natural lens without visually significant cataract, and although it is performed for some higher refractive errors with and without a presbyopia correction IOL, it is typically avoided with low ametropias due to both the greater risk in comparison with laser vision correction and the greater intolerance to multifocality in emmetropic and low myopic patients.
Refractive Surgery of the Cornea or Lens
Laser vision correction to induce monovision, in which one eye is focused for distance and the other for near, is commonly performed for patients in the presbyopic age range. Monovision can also be achieved with monofocal IOLs or contact lenses, and is overall the most popular surgical solution to presbyopia.
Advantages of monovision include spectacle freedom from most daily tasks, a high success rate and the possibility of reversal with enhancement or spectacles. However, stereopsis and contrast sensitivity can be reduced and success relies on neuroadaptation, which is a challenge in highly demanding patients. Additionally, the patient may need glasses for low light conditions, such as night driving.
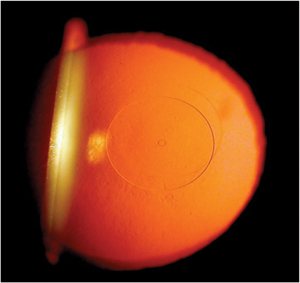 |
| Fig 2. Flexivue microlens within which a 0.5mm central perforation can be seen with retroillumination. Photo: Clark Chang, OD |
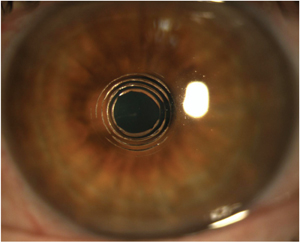 |
| Fig 3. Clinical appearance three hours post-Intracor treatment. Courtesy of Technolas Perfect Vision. |
• Corneal procedures for presbyopia correction are newer and much less common than IOLs. These procedures include presby-LASIK (asphericity modification of the cornea under a flap with an excimer laser), Intracor (femtosecond laser creation of concentric, intrastromal cylindrical incisions) and the implantation of various corneal inlays, which include the following investigational devices: Intracor (Technolas Perfect Vision), Kamra Inlay (Acufocus), Raindrop Inlay (ReVision Optics), Flexivue Microlens (Presbia) and Icolens (Neoptics).2 None of these procedures are currently FDA approved.
Presby-LASIK creates a multifocal cornea by inducing higher-order aberrations that increase depth of focus. Two primary approaches exist: central presby-LASIK and peripheral presby-LASIK.2
Central presby-LASIK creates a steeper myopic corneal center surrounded by a flat hyperopic periphery. This induces negative spherical aberration, which is the most promising aberration used to expand depth of focus. A pinhole effect is created as the pupil constricts with convergence; the central myopic rays are focused through the small pupil, allowing the eye to focus at near. Literature also suggests a faster neuroadaptation response time to this type of aberration.11
In contrast, peripheral presby-LASIK induces positive spherical aberration, which increases pseudo-accommodation. The flattened corneal center focuses distance, while the steepened periphery focuses near. For patient selection, the effects of these aberrations can be simulated using an adaptive optics visual simulator (AOVS). This diagnostic device measures and corrects for pre-existing aberrations, while inducing new aberrations to expand depth of focus and preserve distance visual quality.12 Clinical outcomes for both peripheral and central presby-LASIK show promise, but further studies are needed to investigate long-term stability of the procedure and quality of vision under low contrast settings.2
Intracor creates a hyperprolate, multifocal cornea with femtosecond laser intrastromal incisions in a concentric, cylindrical shaped pattern. Prior to surgery, the line of site is marked using the first Purkinje image. During surgery, five concentric intrastromal rings are cut 2.0mm to 4.0mm from the line of sight.13 These intrastromal circular incisions change the local corneal biomechanics and consequently reshape the cornea. The corneal epithelium and Bowman’s layer remain undamaged during Intracor implantation, allowing the incisions to heal fast.
Although our understanding of the cornea’s biomechanics has grown with advancements in corneal crosslinking, the risk of corneal ectasia still exists after incisional corneal manipulation. In one case report, ectasia developed with an anterior corneal protrusion after hyperopic LASIK followed by Intracor.14 However, another study shows stable corneal steepening and pachymetry at 12 months after Intracor.15
Corneal inlays are currently used in Canada and will likely gain FDA approval soon. The closest to approval is the Kamra, which measures 3.8mm in diameter and 5μm thick with a central 1.6mm aperture. This inlay is implanted in a femtosecond laser-enabled pocket at a depth of 200μm in the nondominant eye, where it reduces the normal aperture for light entry into the eye, thereby creating a pinhole effect and increasing depth of focus.
The Kamra contains laser-etched microscopic openings that maintain metabolic flow to the anterior cornea. These openings are placed in a random pattern to prevent diffraction issues at night. The inlay does not affect examination, and imaging of internal ocular structures and visual field testing shows unchanged thresholds pre- and post-implantation.
Outside of the US, a combined LASIK and Kamra implantation procedure is gaining popularity for the simultaneous treatment of ametropia and presbyopia. In this procedure, the inlay is either placed under a 200µm LASIK flap after excimer ablation or implanted in a pocket deep to a more anterior LASIK flap.2
Two other corneal inlays, the Raindrop and Flexivue, work by central, anterior corneal curvature change and central change in refractive index, respectively. Though rarely used today, conductive keratoplasty is yet another option to induce corneal shape change.
• Lenticular alteration. Experimental presbyopic refractive surgery of the lens is also underway. Intra-lenticular ablation using femtosecond laser, or phacophotomodulation, aims to “soften” the crystalline lens to restore accommodation. In theory, laser microperforations within the hard lens nucleus enhance lens fiber sliding, increasing lens flexure and facilitating accommodation.
In a 2001 study, phacophotomodulation in cadaver eyes reversed age-related loss of lens elasticity.17 Interestingly, other research suggests that femtosecond laser-generated nuclear pinpoint opacities do not cause cataract progression. The same group also created a computer model of the crystalline lens to explore the efficacy of various photodisruption patterns on lens fiber motility.18
Sclerociliary Intervention
The modification of the sclerociliary complex with scleral implants, a blade or laser is also being investigated as an approach to increase accommodation. Although the mechanism is not fully understood and remains controversial, some believe that increased zonular tension causes accommodation.
Scleral implants, such as the PresViews scleral implant (Refocus Group)—a two-piece clear plastic device approximately the size of a grain of rice—increase tension between the lens equator and ciliary muscle, therefore driving accommodation.19 This invasive technique comes with serious risks, however, including migration or extrusion of the implant, scleral perforation, retinal detachment, choroidal or retinal hemorrhage and endophthalmitis. On a more encouraging note, the procedure is extraocular, the visual axis remains untouched during surgery and the implants are removable, meaning the effects may be reversible.
Another developing technology is the LaserAce system (Ace Vision Group), which uses an erbium-YAG laser to create numerous ablation spots through 90% of the scleral depth in an attempt to ease contraction of the ciliary muscle.20
Summary
Just as benefits and setbacks exist with nonsurgical presbyopia correction, surgical techniques—while effective—are far from perfect. Current successful techniques include multifocal and accommodating IOLs, as well as monovision induced by monofocal IOLs or corneal refractive surgery. Several upcoming procedures like corneal inlays and presby-LASIK also show promise and will likely gain FDA approval in the near future.
Other surgeries—including scleral implants, phacophotomodulation and corneal reshaping through intrastromal femtosecond laser ablation—remain investigational and likely require significant advancements before commercialization can be expected.
Regardless, while these techniques each offer varying degrees of success, the ophthalmic and optometric community has yet to develop a sure-fire solution to presbyopia that does not compromise vision or quality of life. A procedure or product that restores accommodation without compromise or risk of adverse effects is in high demand but thus far remains undiscovered.
Dr. Crabb is an associate staff member in the ophthalmology department of the Cleveland Clinic in Ohio.
Dr. Krueger is medical director of the department of refractive surgery at the Cole Eye Institute and a professor of ophthalmology at the Cleveland Clinic.
References
- Ahmad BU, Shah GK, Hardten DR. Presbyopia-correcting intraocular lenses and corneal refractive procedures: a review for retinal surgeons. Retina. 2014;34:1046-1054.
- Waring GO, Berry DE. Advances in the surgical correction of presbyopia. International Ophthalmology Clinics. 2013;53:129-152.
- Gooi P, Ahmed IK. Review of presbyopic IOLs: multifocal and accommodating IOLs. International Ophthalmology Clinics 2012;52:41-50.
- Davison JA, Simpson MJ. History and development of the apodized diffractive intraocular lens. Journal of Cataract and Refractive Surgery. 2006;32:849-858.
- Maxwell WA, Lane SS, Zhou F. Performance of presbyopia-correcting intraocular lenses in distance optical bench tests. Journal of Cataract and Refractive Surgery. 2009;35:166-171.
- Comander J, Pineda R. Accommodating intraocular lenses: theory and practice. International ophthalmology clinics. 2010;50:107-117.
- Yuen L, Trattler W, Boxer-Wachler BS. Two cases of Z syndrome with the Crystalens after uneventful cataract surgery. Journal of Cataract and Refractive Surgery. 2008;34:1986-1989.
- McLeod SD, Vargas LG, Portney V, Ting A. Synchrony dual-optic accommodating intraocular lens. Part 1: optical and biomechanical principles and design considerations. Journal of Cataract and Refractive Surgery. 2007;33:37-46.
- Ossma IL, Galvis A, Vargas LG, Trager MJ, Vagefi MR, McLeod SD. Synchrony dual-optic accommodating intraocular lens. Part 2: pilot clinical evaluation. Journal of Cataract and Refractive Surgery. 2007;33:47-52.
- Bohorquez V, Alarcon R. Long-term reading performance in patients with bilateral dual-optic accommodating intraocular lenses. Journal of Cataract and Refractive Surgery. 2010;36:1880-1886.
- Alio JL, Amparo F, Ortiz D, Moreno L. Corneal multifocality with excimer laser for presbyopia correction. Current Opinion in Ophthalmology. 2009;20:264-271.
- Rocha KM, Vabre L, Chateau N, Krueger RR. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. Journal of Cataract and Refractive Surgery. 2009;35:1885-1892.
- Holzer MP, Mannsfeld A, Ehmer A, Auffarth GU. Early outcomes of INTRACOR femtosecond laser treatment for presbyopia. Journal of Refractive Surgery. 2009;25:855-861.
- Saad A, Grise-Dulac A, Gatinel D. Bilateral loss in the quality of vision associated with anterior corneal protrusion after hyperopic LASIK followed by intrastromal femtolaser-assisted incisions. Journal of Cataract and Refractive Surgery. 2010;36:1994-1998.
- Menassa N, Fitting A, Auffarth GU, Holzer MP. Visual outcomes and corneal changes after intrastromal femtosecond laser correction of presbyopia. Journal of Cataract and Refractive Surgery. 2012;38:765-773.
- Myers RI, Krueger RR. Novel approaches to correction of presbyopia with laser modification of the crystalline lens. Journal of Refractive Surgery. 1998;14:136-139.
- Krueger RR, Sun XK, Stroh J, Myers R. Experimental increase in accommodative potential after neodymium: yttrium-aluminum-garnet laser photodisruption of paired cadaver lenses. Ophthalmology 2001;108:2122-2129.
- Krueger RR, Kuszak J, Lubatschowski H, Myers RI, Ripken T, Heisterkamp A. First safety study of femtosecond laser photodisruption in animal lenses: tissue morphology and cataractogenesis. Journal of Cataract and Refractive Surgery. 2005;31:2386-2394.
- Schachar RA. Theoretical basis for the scleral expansion band procedure for surgical reversal of presbyopia [SRP]. Comprehensive Therapy 2001;27:39-46.
- Swartz TS, Rocha KM, Jackson M, Ma DH, Goldberg D, Hipsley A. Restoration of accommodation: new perspectives. Arquivos Brasileiros de Oftalmologia. 2014;77:V-VII.
- http://www.abbottmedicaloptics.com/products/cataract/refractive-iols/rezoom-multifocal-iol.
