 |
Take Control of the Nutrition Conversation
Comprehensive optometric care includes discerning the role evidence-based nutrition plays in eye health.
By Cecelia Koetting, OD
Release Date: September 15, 2022
Expiration Date: September 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
Discern the role evidence-based nutrition plays in eye health.
Determine how ODs should be involved in the nutrition conversation.
Educate patients on nutrition and its connection to eye health.
Support patients in evidence-based lifestyle changes.
Target Audience: This activity is intended for optometrists engaged in nutrition management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Cecelia Koetting, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #124583 and course ID 80385-GO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Author: Dr. Koetting receives consulting fees from Bruder, Tarsus, Oyster Point, Avellino, Alcon, Abbvie, Glaukos, RVL, Dompé, Claris Bio and Aldeyra. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
For years, there has been discussion around diet and nutrition as it relates to ocular health. Many studies have shown—or suggested—the benefits of certain vitamins and nutrients for a variety of disease states, as well as for overall ocular health. As our patients’ primary eyecare providers, it is important to have a comprehensive understanding of the current literature and its impact on clinical care. This will better equip us to discuss the role of dietary supplements. By working in conjunction with a patient’s primary care physician and other specialists, we can provide a holistic approach to improve our patients’ wellbeing.
The TFOS DEWS II report and the AREDS and AREDS2 studies are three of the larger, well-known efforts that highlight benefits of nutraceutical use in ocular health.1,2 There are many different vitamins and nutrients that are vital for the correct functionality of our eyes, brain and body. However, it is also important to acknowledge that not all nutraceuticals are equal, and any OD discussing nutrition and supplements with their patients must know which brands should and shouldn’t be recommended.
The following is a discussion of the most common ocular disorders, some in relation to systemic disease, where nutraceuticals have been shown by studies to be beneficial. This article will also delve into the role of the OD and how to tackle the nutrition conversation with our patients.
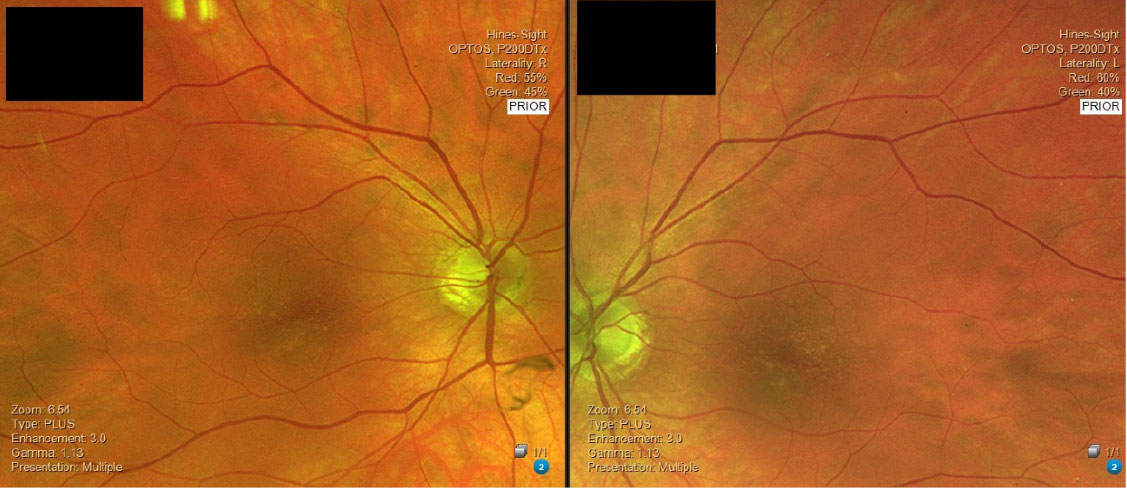 |
| This patient with intermediate AMD was started on AREDS2 vitamins and ForeseeHome monitoring. Click image to enlarge. |
Age-related Macular Degeneration and Retinal Health
Arguably one of the most well-known applications of nutraceuticals to ocular health is the AREDS2 formula, stemming from the AREDS2 study. In the original AREDS study, over 3,000 participants with age-related macular degeneration (AMD) were in one of four groups receiving antioxidant + zinc + copper, zinc + copper, antioxidant formulation only or placebo. The group receiving antioxidant formulation + zinc + copper had the largest reduction in risk for progression from intermediate to advanced AMD and vision loss. Ten years after enrollment, it was found that those who were taking this formula were still 25% to 30% less likely to develop advanced AMD than those in the placebo group.2
In 2006, the AREDS2 study was started to test the original formula while adjusting zinc levels and adding either omega-3 fatty acids or lutein + zeaxanthin to see if there was an increase in efficacy.2,3 At the same time, the study tested the effect of eliminating beta-carotene, which had become a concern for increased risk of lung cancer in smokers.2,3 Over 4,000 patients with either intermediate or severe AMD (in one eye) were enrolled. No patients without AMD or with early AMD were included in AREDS2 since the original formula was not shown to be beneficial for these patients.
Both the omega-3 and lutein + zeaxanthin arms demonstrated no significant added risk reduction for AMD advancement. It was noteworthy that those in the arm receiving antioxidants with lutein + zeaxanthin but without beta-carotene showed an incremental increase in benefit over those who took the original AREDS formula. This became what we now know as the AREDS2 formula (Table 1).2,3
In June 2021, the results of the 10-year follow-up study of AREDS2 were released. The study focused on assessing the long-term effect of adding lutein, zeaxanthin and omega-3 fatty acids to the original AREDS on AMD progression and adverse side effects.4 A total of 6,460 eyes (3,887 participants) were followed, and the study found that 58% of these patients progressed to late AMD.4 This indicated that the 10-year findings replicated findings of the five-year AREDS2 trial.4 Both lutein and zeaxanthin had incremental beneficial effect on progression to late AMD compared with beta-carotene, which doubled the risk of lung cancer.4
Diabetes and Ocular Health
The leading global cause of preventable blindness in the working-age population is diabetes, affecting an estimated 425 million adults worldwide in 2019.5,6 The major causal factors leading to retinal vascular damage and neovascularization in people with diabetes are oxidative stress and inflammation.6 It is believed that oxidative stress pushes reactive oxygen species into mitochondrial overproduction within the retina. This leads to disruption of cellular signals and cellular damage.10
The optometrist’s discussion with diabetic patients includes optimizing glycemic control. There is a direct correlation with poor control in blood sugar and progression of diabetic retinopathy. Many of us feel comfortable discussing diet and changes that can be made, or at the very least referring to a dietician. However, this should not be where the conversation ends.
Another thing that should also be discussed is appropriate dietary supplementation in patients who are known to have poor dietary habits. One supplement that is widely discussed within eye care is omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Both of these are long-chain n-3 polyunsaturated fatty acids, which in studies have been used as a therapy for retinal diseases.6,8,9 This is, in part, due to their pleiotropic effects which include anti-inflammatory, antioxidant, antiproliferative and antiangiogenic properties.6,8,9 DHA is important for function of the retinal vascular and sensory systems; EPA is converted into DHA within our cells.6,8,9 Dietary consumption of DHA or oily fish has been shown to have a protective role against diabetic retinopathy.10
The PREDIMED trial, a large prospective cohort study, sought to further investigate this association.10 While the original study was widely criticized due to protocol deviations, it was retracted and republished in June 2018. The trial included 3,482 patients, all of whom at baseline had a previous diagnosis of type 2 diabetes. It investigated the risk of diabetic retinopathy in relation to dietary DHA/EPA intake.10
At baseline, 75% of the patients were meeting the recommended intake of 500mg per day of DHA/EPA, equivalent to two weekly servings of oily fish, had a lower prevalence of hypertension and were being treated with insulin.10 After screening, all patients were assigned to either a Mediterranean diet with extra virgin olive oil, a Mediterranean diet with nuts or a control diet reducing all dietary fat.10
At the six-year follow-up, those who reported diets with an intake of at least 500mg per day of EPA/DHA at baseline had a 46% decreased risk of sight-threatening diabetic retinopathy.10 Patients who did not meet that intake at baseline but were assigned to the Mediterranean diet with extra virgin olive oil had a 63% decreased risk of sight-threatening diabetic retinopathy.10 Overall, these were very interesting outcomes, suggesting that increased dietary DHA/EPA, possibly with extra-virgin olive oil or with two servings of oily fish per week, can decrease the risk of development or worsening of diabetic retinopathy.10
Multiple studies have found that omega-3 dietary supplements with both high EPA and DHA provide a higher antioxidant effect on human retinal cells, increasing cell viability and proliferation while also repairing oxidative-induced damage to retinal pigment epithelium cells.6,8 In diabetic patients, this may help to prevent or delay diabetic retinopathy.
Omega-3 fatty acids are also helpful for corneal function in diabetic patients. One study found that patients with type 1 diabetes receiving 1,800mg per day of oral omega-3 fatty acid supplements for 180 days exhibited corneal neuro-regenerative effects when compared with placebo.9 This was measured by an increase in central corneal nerve fiber length and corneal sensitivity at the end of follow-up compared with baseline.9
Another vitamin that is important to retinal function is vitamin D. This fat-soluble vitamin plays a role in the retina as an antioxidant, anti-inflammatory and anti-angiogenic in patients with diabetes.11 It helps to decrease production of pro-inflammatory cytokines and helper T-cells, cytotoxic T-cells and natural killer cells.11 Previously it has been found to be involved with vascular endothelial dysfunction leading to compromise of the blood retinal barrier.11
DiVFuSS, a small study of 67 patients, investigated the use of a novel multi-component formula in patients with type 1 or 2 diabetes with no retinopathy or mild to moderate nonproliferative retinopathy.12 Interestingly, patients with diabetic macular edema were also included.12
The formula contained vitamin C, D3 and E, zinc oxide, EPA, DHA, alpha lipoic acid, coQ10, mixed tocotrienols/tocopherols, zeaxanthin, lutein, benfotiamine, N-acetyl cysteine, grape seed extract, resveratrol, turmeric root extract, green tea leaf and pycogenol.12 Patients were given this formula or placebo canola oil soft gel twice a day.12
After six months of use, those who had been on the supplement had significantly better visual function as well as an improvement in serum lipid levels and peripheral neuropathy.12 No significant change was found in retinal nerve fiber layer thickness, total cholesterol or HbA1c.12 While the study size was small, it supports evidence of improvement in visual function among other things for patients with and without nonproliferative diabetic retinopathy, warranting further investigation.12
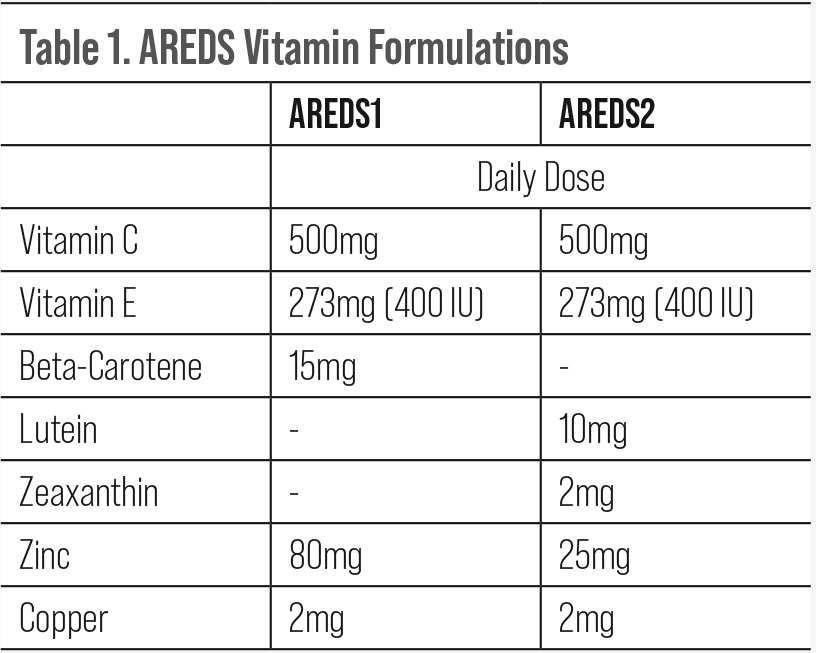 |
| Click table to enlarge. |
Corneal Disease and Ocular Surface Disease
Vitamin D is also important for corneal function and has been shown to control inflammation in both corneal healing and chronic ocular surface disease. In addition to this, vitamin D also stimulates expression of antimicrobial peptides, which is beneficial in the presence of infection and wound healing.13 In association with dry eye disease (DED), one study looked at the level of serum vitamin D and tear fluid and found both to be lower in patients with DED.14 Tear vitamin D levels were more closely associated with DED and ocular surface discomfort, in theory because they regulate the expression of inflammatory cytokines and protect the corneal epithelial barrier function. All of this is in favor of enhancing corneal and tear vitamin D levels.
Vitamins C and A are well-known for their roles in corneal regulation and healing. Vitamin A specifically helps in epithelial growth and limbal stem cell differentiation.15 Vitamin A ophthalmic ointment or oral supplements can be used to help speed epithelial healing.15 Improved corneal wound healing and decreased corneal scarring post-surgery have been associated with use of oral vitamin C. This is in part due to the important role vitamin C plays in enhancing collagen synthesis and suppressing corneal neovascularization.16
One study looked at reduction of corneal opacity with use of vitamin C supplementation in patients with infectious keratitis.16 It found that in conjunction to antibiotic therapy both oral and intravenous vitamin C supplementation helped with healing the cornea and reducing the corneal opacity.16 Additionally, intravenous vitamin C decreased the corneal opacity size more so than oral.16
Over the last two decades, the role of omega-3 fatty acid supplementation in DED and meibomian gland dysfunction has been well discussed. In 2005, the Women’s Health Study of over 32,000 women showed an association between DED and diets low in omega-3 fatty acids.17 Confirmation has been demonstrated in many studies. A meta-analysis in 2019 demonstrated significant improvement in signs and symptoms of DED with omega-3 use.18
At this time, omega-3 fatty acids are considered part of first-line therapy for DED with a recommended dosage of 2g of EPA/DHA at a 3:1 ratio.1,19-22 Not all omega-3 fatty acids are created equal, and most OTC options are synthetic or an unpurified form that is poorly absorbed. This is a partial cause of the fishy taste and odor. Concern for elevated heavy metals such as mercury within supplements has been reported by consumerlab.com, an independent nutraceutical quality testing company, to be less than that in fresh fish meat. This is consistent with a 2018 study published that found extracted mercury levels were two to three times lower than that found in fish.23
The DREAM study in 2018 had some controversial conclusions, stating that there were no beneficial effects of taking omega-3 supplements over the placebo, which was olive oil. However, what it did show was a statistically significant improvement in the OSDI score in both groups, with no statistically significant difference between the groups.24 Larger studies looking at the use of olive oil and Mediterranean diet would be worth investigating.
Another study, VITAL, that challenged the use of omega-3 fatty acids for DED was published in JAMA Ophthalmology in July 2022.25 The original study looked at the use of 2,000 IU of vitamin D3 and/or marine omega-3 fatty acids at 1g daily in prevention of cancer and cardiovascular disease in 25,871 adults.25 The follow-up reviewed the data collected from the original study participants, excluding anyone who previously had a diagnosis of DED or severe symptoms of dry eye based on patient questionnaires.25 It is important to understand that this study was specifically looking at preventing DED.25
The findings showed that when the group taking marine omega-3 only was compared with the omega-3 placebo group, the same amount of patients developed dry eye.25 The incidence of DED was 0.7% in the placebo group and 0.7% in the omega-3 group in those 50 to 65 years old and 1.24% in placebo vs. 1.16% in omega-3 in patients over 65.25 As we know, DED is multifactorial and not solely reliant on one treatment, with incidence increasing with age, so the findings are not necessarily surprising.
Some limitations regarding this study exist. The omega-3 fatty acid used was EPA and DHA in a 1.2:1 ratio, which is significantly lower than that suggested (3:1) for use in DED, and at a lower dosage of 1g vs. the suggested 2g.1,19-22,25 None of the included patients had DED to start as determined by a questionnaire.25 Only when symptoms were reported on the follow-up questionnaire one year later were records from the patient’s eyecare physician obtained.25 Lastly, the contents of the placebo omega-3 were not published, so it could be that this was another oil that contains some fatty acid benefits.
Vitamin B12 deficiency has also been evaluated in both causality and treatment among patients with severe DED and neuropathic ocular pain (NOP). In a study of 90 patients, severe DED and ocular pain were divided into two groups. The first included patients with both severe DED and vitamin B12 deficiency receiving parenteral vitamin B12 supplement, topical artificial tears and cyclosporine. The second group, with severe DED and normal vitamin B12 levels, received only topical artificial tears and cyclosporine.26
Both arms were evaluated with OSDI, tear breakup time (TBUT) and Schirmer’s type 1.26 The vitamin B12 level increased in group one after 12 weeks of treatment, as did the mean TBUT and Schirmer’s score, and the OSDI questionnaire score significantly dropped.26 Group two also had improvements in these areas; however, it was not as profound.26 This may indicate that we should be considering vitamin B12 levels in our patients with NOP and severe DED.
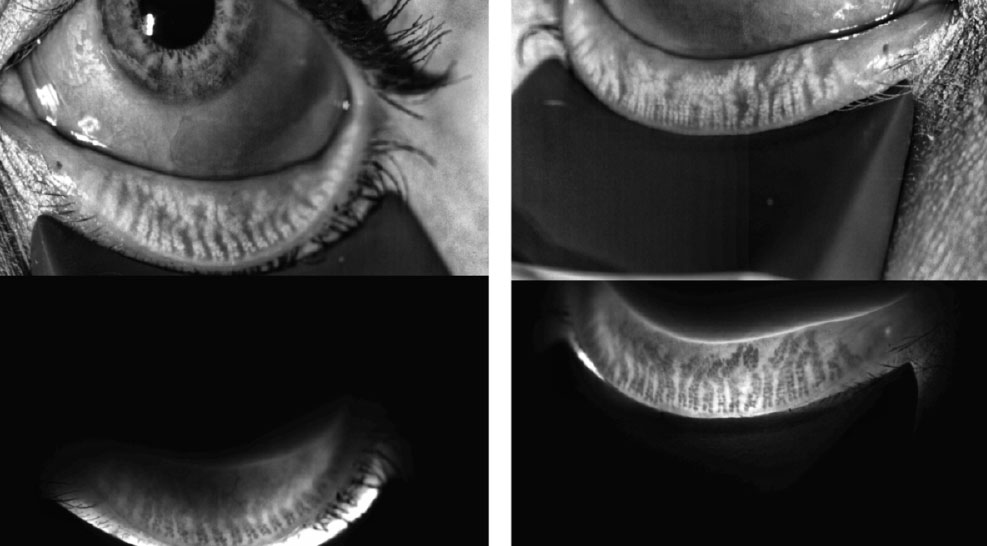 |
| This patient with minimal atrophy, moderate inspissation and loss of gland structure OD>OS was started on omega-3 fatty acids and had thermal pulsation. Click image to enlarge. |
Exercise, BMI and Ocular Health
Regular aerobic exercise and lower BMI are known for their benefits to our body and minds overall but have also been shown to be beneficial for our ocular health. Greater cataract risk has been found in patients with greater inactivity, and vice-versa.27,28 Decreased risk of progression in patients with primary open-angle glaucoma and lower IOP has been documented.29
Exercise has been shown to have a protective effect on progression of dry to wet AMD.30 Increased risk has been found for progression of dry to wet AMD in those with overall abdominal obesity.31 In regard to the Mediterranean diet, patients with AMD may benefit from a reduction in their dietary glycemic index, especially those with diabetes.32,33 This is just one more reason it is important for us as healthcare professionals to discuss a healthy diet and regular exercise with our patients.
Eating Habits and Nutraceuticals
Diet is the best source for nutrients and vitamins. Our bodies readily absorb these better than supplements. Non-processed and fresh foods carry more nutrients and vitamins, which are decreased in the milling and storage process.34 Some of the best sources for dietary vitamin A are in green, orange and yellow vegetables (e.g., carrots and spinach).35 Vitamin D is not naturally occurring in many foods other than fatty fish like salmon and tuna but has been fortified in most cow milk within the United States.35
The same can be said for omega-3 fatty acids. Fatty fish and plant oils are the most common, but products like eggs, milk and yogurt can be found fortified with omega-3 fatty acids.35 Vitamin B12 is found mainly in animal products and some seafood or shellfish, again with other foods that may be fortified.35 Lastly, and arguably the easiest, is vitamin C. Found in citrus fruits, juices, red and green peppers, broccoli, kiwi, strawberries, potatoes and tomatoes, there is a little something for everyone.35
According to CDC data from the National Health and Nutrition Examination Survey, 57.6% of adults over 20 years of age in the United States were taking dietary supplements in 2017 and 2018.36 Use was higher among women than men and increased with age, highest among women 60 years and older at 80.2%.36 The most commonly used dietary supplements were multivitamin mineral supplements, followed by vitamin D and omega-3 fatty acids.36
Use of dietary supplements and vitamins are good ways to help improve our dietary deficits. However, the body’s ability to absorb these varies based on chemical composition. This can also be affected by surgeries that alter our digestive system such as ostomies or bariatric surgery.37 With a decreased surface area for absorption either with the colon or the stomach, the most common vitamin deficiencies are B12, iron, calcium, vitamin D and calcium.37
Which Supplements to Use?
It is important for optometrists to discuss treatment options with patients, including diet, exercise and nutraceuticals. When adapting new knowledge and practice habits, this can take time and research.
There are different approaches and steps that can be taken to help our patients. Initiating discussions around nutrition and eye health should be the bare minimum. From here you can refer the patient to a dietician as needed or provide direction on what exactly they need to add to their diet or supplement intake.
Quality vs. quantity applies when talking about vitamins and supplements. What is on the label may in fact not always be correct when it comes to the true content. Before recommending products, or taking them ourselves, it is important to verify any marketing claims. The FDA does not have the authority to approve dietary supplements, and companies do not have to provide evidence to substantiate safety of their products, unless they contain a new dietary ingredient.38 This is defined as a dietary ingredient that was not marketed in the United States in a dietary supplement before October 15, 1994.38 While companies do not have to submit to the FDA, under the Federal Food, Drug and Cosmetic Act companies are held responsible for ensuring that dietary supplements are not misbranded or providing false claims of function.38 Companies do not have to submit prior to putting claims on their products such as “supports better vision” or “helps lose that belly fat;” however, if challenged, they are responsible for providing documentation and research backing these claims.
This is why many nutraceutical companies have independent certification labels, such as Natural Products Association (NPA), US Pharmacopeial Convention (USP) and NSF certified. This allows a third party to test for purity and safety of their product. Beyond this, there are independent groups such as labdoor.com and consumerlab.com that test the quality and content of products. The ConsumerLab website also provides information on who the FDA has flagged regarding false claims of content and function.
When considering the supplements and vendors that we may use as eyecare professionals, there are many choices. Don’t hesitate to ask about independent research or white papers that may help you when deciding which you prefer to recommend. Several eyecare companies have a wide selection of eye health formulas and have taken the time to make sure their research materials are available for both patients and physicians.
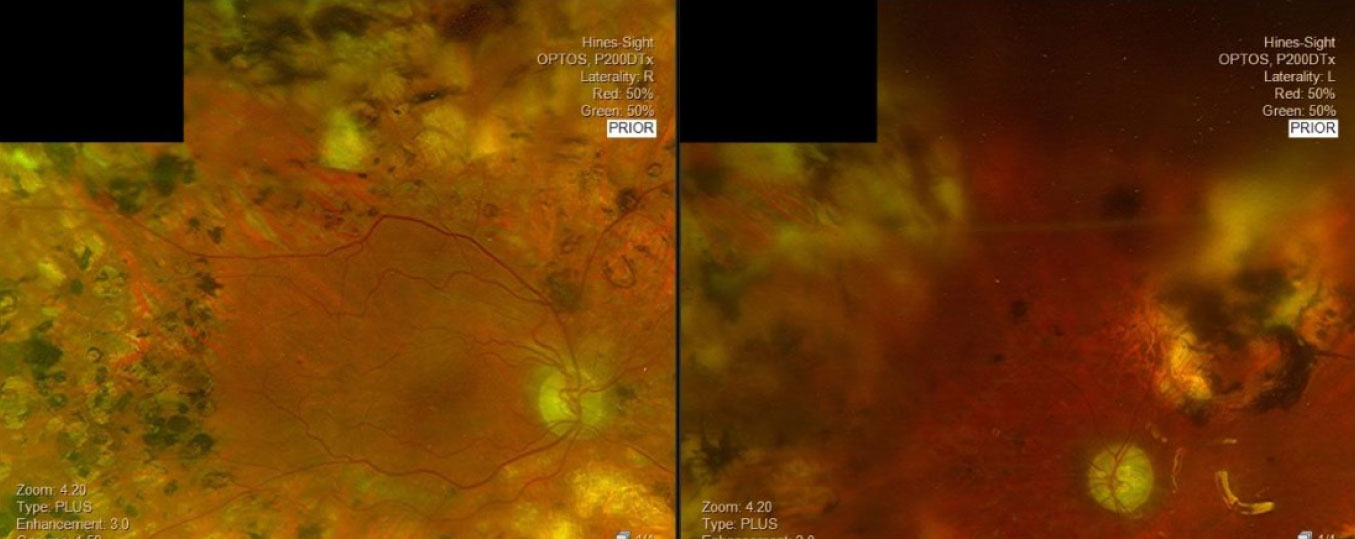 |
| This patient with poorly controlled diabetes and a history of proliferative diabetic retinopathy and panretinal photocoagulation OU initiated omega-3 fatty acid treatment with lutein and zeaxanthin. Click image to enlarge. |
To Sell or Not to Sell?
There are varying opinions among eyecare professionals regarding both use and in-office sales of nutraceuticals. It is important to remember that these products are not cures but serve as tools or other options to supplement treatment for our patients. The science and benefits have already been discussed but the pros and cons of in-office sales should also be considered.
Carrying supplements in-office or working with different nutraceutical companies for direct-to-patient sales can be beneficial for both the patient and the practitioner. Patients are much more likely to follow through with treatment plans when there is a firm recommendation on which products to use, why and how. When they know that something is being prescribed vs. suggested, it changes compliance.
Recommend one or two specific brands, tell the patient where to find them and the exact dosage they need. Taking it a step further and carrying nutraceuticals in-house or sending in a prescription to the company puts the product in the patient’s hand, and they are much more likely to follow through with use. Many companies have auto-renewal and shipment options as well, similar to mail order pharmacies. This ensures the patient will not run out and will be able to continue with treatment.
This can also be financially beneficial for the practice as well as the patient. Selling products within the office can lead to a passive income, and many of companies offer a percentage of online sales when you set up an account to allow your patients to order. This presents an option for those who do not have the footprint to offer products in-house or those who work in a retail setting or partnership where they may not have the ability to make these executive decisions. However, none of these options will work if we as doctors do not take the time to discuss the importance and value of this approach with our patients.
As with any in-office sale, there is a concern that the patient may perceive that we are only trying to make money. This may be reason enough for some to avoid in-office sales, but it should not mean that we don’t take an active role in the nutrition conversation.
The correct dietary balance of important nutrients and vitamins is vital for the functionality of our body, including our eyes. Educating our patients regarding both dietary changes and supplements should be part of our exam. As members of the healthcare team, optometrists need to be prepared to aid in the continued discussion regarding diet and exercise with patients as a part of a more holistic approach.
Dr. Koetting practices at the MD/OD practice Hines Sight in Denver. Her primary focus is anterior segment and ocular surface disease, neuro-optometry and perioperative care. She is a fellow of the American Academy of Optometry, a diplomate of the American Board of Optometry, an active member of the American Optometric Association (AOA) and has served as both local and state officers of the AOA. She receives consulting fees from Bruder, Tarsus, Oyster Point, Avellino, Alcon, Abbvie, Glaukos, RVL, Dompé, Claris Bio and Aldeyra.
1. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 2. National Eye Institute. AREDS/AREDS2 clinical trials. www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-studies-aredsareds2/about-areds-and-areds2. Accessed 7/10/22. 3. Chew EY, Clemons TE, Sangiovanni JP, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmology. 2014;132(2):142-9. 4. Chew EY, Clemons TE, Keenan T, et al. The results of the 10 year follow-on study of the age-related eye sisease study 2 (AREDS2). Invest Ophthalmol Vis Sci. 2021;62(8):1215. 5. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1234. 6. Saenz de Viteri M, Hernandez M, Bilbao-Malavé V, et al. A higher proportion of eicosapentaenoic acid (EPA) when combined with docosahexaenoic acid (DHA) in omega-3 dietary supplements provides higher antioxidant effects in human retinal cells. Antioxidants (Basel). 2020;9(9):828. 7. Valle, Maria Stella, Cristina Russo, and Lucia Malaguarnera. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes/Metabolism Research and Reviews 37.8 (2021): e3447. 8. Xiao Y, Zhang Q, Liao X, et al. The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2022;182:102456 9. Alexis Britten-Jones AC, Kamel JT, Roberts LJ, et al. Investigating the neuroprotective effect of oral omega-3 fatty acid supplementation in type 1 diabetes (nPROOFS1): A Randomized Placebo-Controlled Trial. Diabetes. 2021;70(8):1794-1806. 10. Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 2016;134(10):1142-9. 11. Valle MS, Russo C, Malaguarnera L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab Res Rev. 2021;37(8):e3447 12. F Chous AP, Richer SP, Gerson JD, et al. The diabetes visual function supplement study (DiVFuSS). Br J Ophthalmol. 2016;100:227-34. 13. Reins RY, Hanlon SD, Magadi S, et al. Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016;11(4):e0152889 14. Khamar P, Nair AP, Shetty R, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60(7):2532-42. 15. Chelala E, Dirani A, Fadlallah A, et al. The role of topical vitamin A in promoting healing in surface refractive procedures: a prospective randomized controlled study. Clin Ophthalmol. 2013;7:1913-8. 16. Cho YW, Yoo WS, Kim SJ, et al. Efficacy of systemic vitamin C supplementation in reducing corneal opacity resulting from infectious keratitis. Medicine (Baltimore). 2014;93(23):e125. 17. Ziemanski JF, Wolters LR, Jones-Jordan L, et al. Relation between dietary essential fatty acid intake and dry eye disease and meibomian gland dysfunction in postmenopausal women. Am J Ophthalmol. 2018;189:29-40. 18. Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea. 2019;38(5):565-73. 19. Lum F, Feder RS, McLeod SD, et al. The preferred practice pattern guidelines in ophthalmology. Ophthalmology. 2016:123(5):928-9. 20. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl 1):3-47. 21. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-84. 22. Akpek EK, Amescua G, Farid M, et al. Dry eye syndrome preferred practice pattern. Ophthalmology. 2019;126(1):286-P334. 23. Mei N, Bunhong L, Liu J, et al. Speciation of trace mercury impurities in fish oil supplements. Food Control. 2018;84:221-5. 24. Asbell PA, Maguire MG, Peskin E, et al. Dry eye assessment and management (DREAM) study: study design and baseline characteristics. Contemp Clin Trials. 2018;71:70-9. 25. Christen WG, Cook NR, Manson JE, et al. Efficacy of marine ω-3 fatty acid supplementation vs placebo in reducing incidence of dry eye disease in healthy US adults: a randomized clinical trial. JAMA Ophthalmol. 2022;140(7):707-14. 26. Ozen S, Ozer MA, Akdemir MO. Vitamin B12 deficiency evaluation and treatment in severe dry eye disease with neuropathic ocular pain. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1173-7. 27. Williams PT. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50:95-100. 28. Selin JZ, Orsini N, Lindblad BE, et al. Long-term physical activity and risk of age-related cataract: a population-based prospective study of male and female cohorts. Ophthalmology. 2015;122:274-80. 29. Tsai JC. Influencing ocular blood flow in glaucoma patients: the cardiovascular system and healthy lifestyle choices. Can J Ophthalmol. 2008;43:347-50. 30. Knudtson MD, Klein R, Klein BEK. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. 2006;90:1461-3. 31. Seddon JM, Cote J, Davis N, et al. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785-92. 32. Merle BMJ, Silver RE, Rosner B, et al. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015;102:1196-206. 33. Chiu C, Milton RC, Gensler G, et al. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86:180-8. 34. Rock CL. Multivitamin-multimineral supplements: who uses them. Am J Clin Nutr. 2007;85(1):2778-95. 35. National Institutes of Health. https://ods.od.nih.gov/factsheets/list-all/#A. Accessed 7/20/22. 36. Centers for Disease Control and Prevention. www.cdc.gov/nchs/products/databriefs/db399.htm. Accessed 7/10/22. 37. Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006 Apr;331(4):219-25. 38. U.S. Food and Drug Administration. www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements. |
