 |
Should You Prescribe a Glaucoma Drug For Ocular Hypertension?
Knowing when intervention is and isn’t warranted is a key component of comprehensive care.
By Brian D. Fisher, OD, Anne Menjivar, OD, Danielle Howard, OD, Michelle Nguyen, OD, and Elyse Banister, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: April 15, 2023
Expiration Date: April 15, 2026
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists engaged in ocular hypertension management.
Educational Objectives: After completing this activity, participants should be better able to:
- Determine when to prescribe a glaucoma drug for ocular hypertension.
- Evaluate an ocular hypertension patient’s overall risk profile.
- Educate patients on their individual risks and needs.
- Manage patients with ocular hypertension.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Drs. Fisher, Menjivar, Howard, Nguyen and Banister have nothing to disclose. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #125805 and course ID 83915-GL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
Glaucoma is one of the leading causes of irreversible blindness in the United States—and worldwide. There are 80 million people globally with glaucoma, and this number is expected to increase to over 111 million by 2040.1 In the United States, more than three million Americans are living with glaucoma, and 2.7 million aged 40 and older are affected by primary open-angle glaucoma (POAG).
Ocular hypertension (OHT) is another common condition clinicians can encounter and is considered a risk factor for conversion to POAG. While OHT’s diagnosis can be forthright with intraocular pressures (IOPs) greater than 21mm Hg, normal optic discs and normal visual fields, management can become quite complex and variable depending on the experience level of the clinician.2 These management challenges occur in OHT patients due to glaucoma’s long latency phase, in which glaucomatous optic nerve damage has started, but the disease can remain undetectable on ancillary testing and the patient, asymptomatic.3
As optometrists, we should consider the following viewpoints to optimize our clinical decisions in treating our patients: (1) detecting early to prevent functional vision impairment and disability, (2) maintaining visual abilities for our patients to live independently and stay physically active, (3) reducing psychological stress and (4) negating the medication and medical costs.
The decision to treat depends on a variety of ocular, systemic, medical and psychosocial factors. Additional factors such as life expectancy, general health status and perceptions and/or expectations about treatment should also be considered.3 There is less universal agreement about treating OHT in its earlier stages or suspects without clear structural or functional damage.4
The OHTS clinical trial—one of the largest (1,696 patients) and longest (20 years) studies to date—aimed to address this issue in phases I through III. Here, we will discuss all three and how the results of each can help guide our clinical decision-making in OHT.
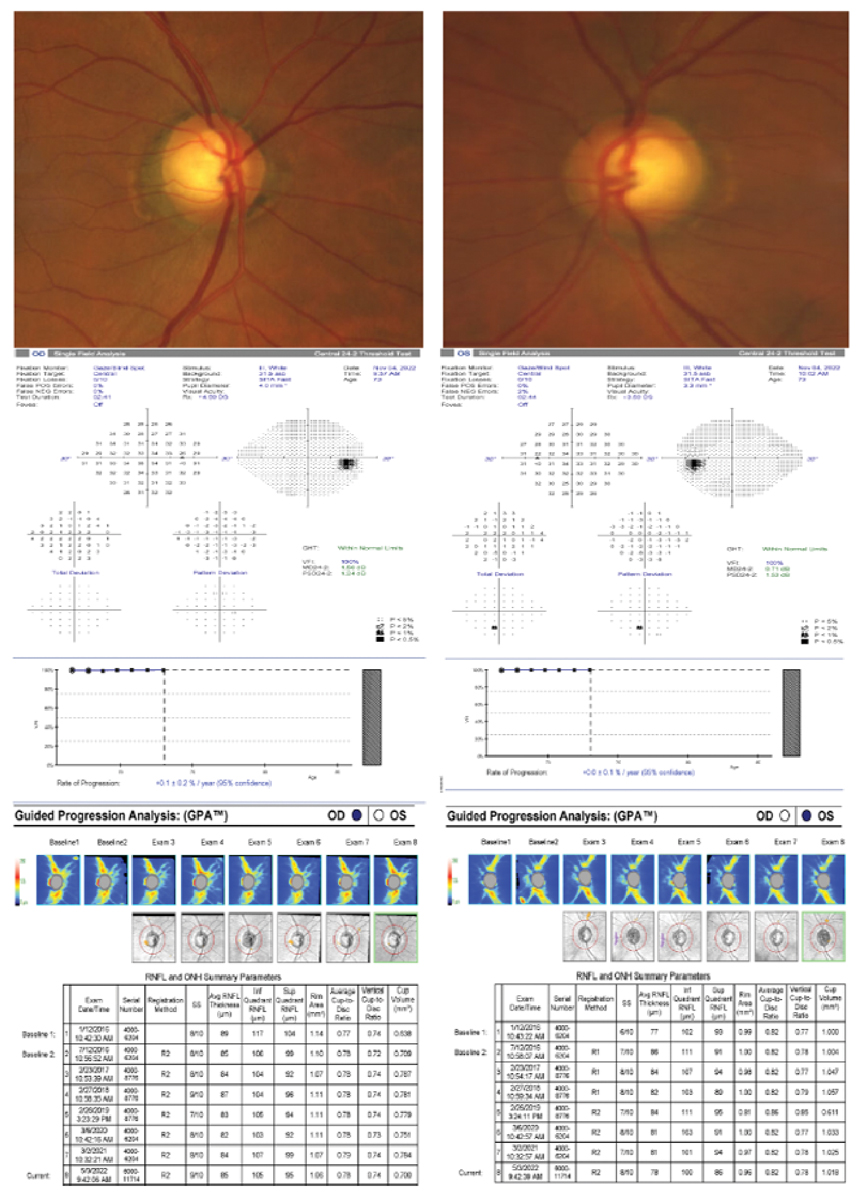 |
A 71-year-old Hispanic male with OHT, obstructive sleep apnea and a strong family history of glaucoma. His untreated IOP max was 26mm Hg in both eyes, pachymetry measured 576µm OD and 577µm OS, VCDR was 0.70 OD and 0.75 OS and PSD was 1.24 OD and 1.53 OS. Toward the end of the two years of observation, his untreated IOPs started to slowly rise. He was started on prostaglandin analogue treatment after year two due to moderate risk factors including rising untreated IOPs, VCDR, race, obstructive sleep apnea, positive family history, general health status and anticipated life expectancy. Early intervention in this case was effective in preserving structure and function as shown in the visual field and RNFL progression analyses. Click image to enlarge |
OHTS-I
Each of the three OHTS phases has provided significant evidence about early treatment and observation of OHT, delayed treatment of OHT, incidence and severity of OHT, conversion to POAG and conversion prediction models.
OHTS-I randomized 1,636 OHT participants into a medication group and close observation group. Data collection started in 1994 and finished in June 2002. The clinicians in OHTS-I could use any topical OHT medication approved for use in the United States. Of note, topical beta-blockers were considered first-line topical treatment when the study began, and prostaglandin analogues were not introduced commercially until 1996. Twenty-five percent of the participants self-identified as African American.5 The main goals of OHTS-I were to achieve an IOP of 24mm Hg or less and a minimum reduction of 20%. Others included identifying the safety and efficacy profiles of using topical OHT medication and predicting which patients would convert to POAG.
After 60 months, the cumulative frequency of developing POAG was 4.4% in the medication group and 9.5% in the observation group. These results indicated a positive therapeutic effect that was statistically significant for both optic disc and visual field protection. The absolute risk reduction of 5.1% indicated having to treat 20 OHT patients to prevent one from converting to glaucoma. No evidence of ocular- or systemic-associated risk was found. Notably, most of the early glaucomatous change was seen on optic disc photographs, while one-third of participants had their initial glaucomatous change represented on visual fields.6
Optic disc damage was classified as a change in the position of the vessels greater than would be expected from a shift in the position of the eye, development of a notch, development of an acquired pit and/or overall thinning of the rim. Though disc hemorrhages, localized RNFL dropout or changes in the depth of the cup were not considered evidence of optic disc change in OHT, they should still be considered as risk factors for glaucoma in all patients. Visual fields were considered abnormal if the pattern standard deviation (PSD) was p <5% or if the glaucoma hemifield test (GHT) was outside normal limits.
OHTS-I also successfully identified the clinical risk factors that could predict conversion from OHT to POAG, which have been widely accepted as a predictive model in separating patients into high- and low-risk categories. Older age, higher baseline untreated IOP, greater PSD, thinner central corneal thickness (CCT) and larger vertical cup-to-disc ratio (VCDR) were identified as significant predictors of the development of POAG.
Several of these risk factors were further broken down into the following categories:
- IOP: ≤23.75mm Hg, >23.75≤25.75mm Hg and >25.75mm Hg.
- CCT: ≤555µm, >555µm≤588µm and >588µm.
- VCDR: ≤0.3, >0.3≤0.5 and >0.5.
A thin CCT (≤555µm) was associated with a threefold increase in POAG as compared with participants with a thick CCT (>588µm), and this was found to hold true regardless of which category the baseline IOP and VCDR fell under.7 There was a linear relationship between CCT and conversion to POAG, and CCT was shown to be a strong predictor of POAG. A greater PSD was noted to be predictive of POAG and was calculated by averaging PSD from multiple Humphrey 30-2 visual field tests. These factors were confirmed in several other studies.8,9
African Americans enrolled in the study were also at higher risk of conversion to POAG, but race was dropped from the OHTS-I predictive model. Once multivariate analysis was used to adjust for thin CCT and large VCDR, race no longer showed any statistical significance.10
 |
|
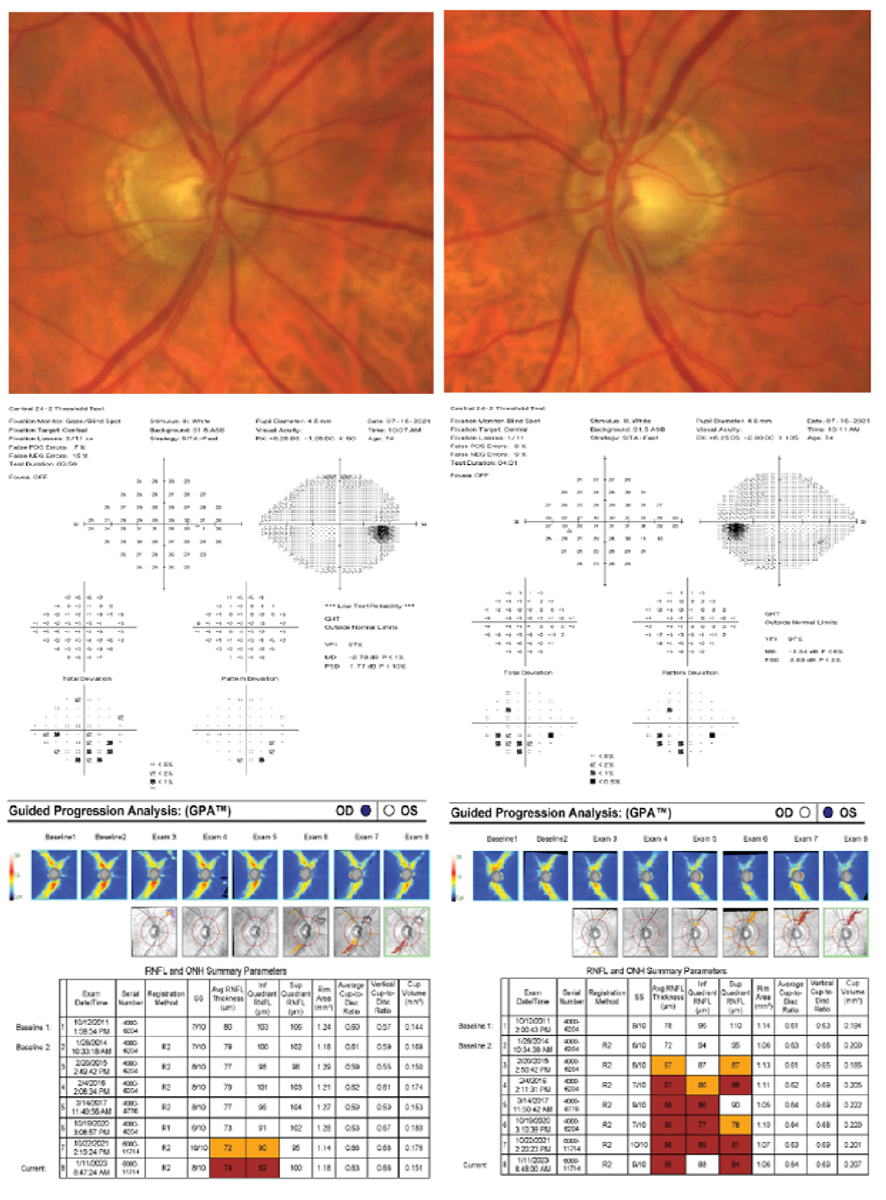
A 75-year-old white, non-Hispanic male presented with an initial history of OHT, but years of non-compliant follow-up and medication use resulted in accelerated loss of the RNFL and ultimate conversion to POAG. His untreated IOP max was 28mm Hg in both eyes, pachymetry measured 527µm OD and 529µm OS, VCDR was 0.60 OD and 0.70 OS and PSD was 1.77 OD and 2.53 OS. His visual fields were variable in both eyes. His RNFL showed accelerated and repeatable loss OS>OD. His deviation maps showed a widening wedge defect strongly correlated with glaucomatous RNFL loss. Based on these high-risk factors, medication and selective laser trabeculoplasty (SLT) were recommended at the time of diagnosis. The patient declined and experienced both structural and functional damage. Luckily, over the last three years with SLT and compliant topical prostaglandin analog use, his treated IOPs were lowered by 50%. This ultimately preserved his visual function without sustainable severe glaucomatous defects. Due to his general health status and anticipated life expectancy, he remains at risk for further visual damage if compliance and medication burden remain an issue. Click image to enlarge. |
OHTS-II
Results from OHTS-I showed early treatment was successful in decreasing the incidence of and conversion to POAG without adverse ocular or systemic effects. OHTS-II aimed to identify when treatment should be initiated.
There are several approaches and factors a clinician can face when determining the best course of treatment. These include: (1) treat every patient with elevated IOP, (2) defer treatment until there is detectable optic disc or visual field damage or (3) selectively treat patients identified as moderate to higher risk for conversion to POAG. Treating every patient in public health with OHT can be costly in both medications and office visits. The patient’s quality of life can be affected due to medication burden and pressure to be compliant.
Though serious adverse reactions are atypical for prostaglandin analogues and other topical ocular hypotensive drug classes approved for use in the United States, we should remain aware of the systemic side effects of topical beta-blockers. Contraindications include patients with a history of asthma or a chronic obstructive pulmonary disease, bradycardia, heart block or uncontrolled heart failure. Side effects from the other topical medication classes include, but are not limited to, ocular surface disease, hyperemia, cosmetic and pigmentary changes from prostaglandin analogues and sulfa allergy from carbonic anhydrase inhibitors. The potential benefit of treatment resulting in a low POAG conversion rate should outweigh the alternatives, consequences and risks of no treatment.
In opposition, one can choose to delay treatment and wait for optic disc and/or visual field damage, but this could lead to accelerated retinal nerve fiber degeneration that is less responsive to treatment. This approach could cause visual impairment and result in a major public health issue if universally adopted. The best approach to treatment should address our viewpoints discussed earlier and, most importantly, ensure the prevention of functional vision impairment and disability and maintain our patients’ visual abilities.11
OHTS-II aimed to identify if the cumulative incidence of POAG was greater in the delayed treatment group and to determine if the subsequent course of treatment after diagnosis was worse in this group. The same participants in the treatment and observation groups from OHTS-I were followed for an additional 7.5 years. The treatment group participants remained on treatment for an additional 5.5 years, and the observation group from OHTS-I received topical OHT treatment for 5.5 years. This essentially created a treatment group (13 years) and a delayed treatment group (7.5 of observation and 5.5 years of treatment). Tests, measures and procedures remained consistent in both phases of the study.
Results from OHTS-II showed the median time to develop POAG was six years in the delayed treatment group and 8.7 years in the medication group, demonstrating a positive protective effect of early treatment.12 After the study, participants were divided into equal distributed groups of high, medium and low risk using the predicative model from OHTS-I.
In determining the management effect of delayed treatment, the clinical course after diagnosis of POAG should be evaluated in both treatment groups. The data from these phases showed modest consequences for delaying treatment in OHT participants. Consequences were less tolerable in the high-risk group compared with the low-risk group, and we can conclude high-risk individuals benefit from earlier treatment and more frequent follow-up examinations.
The disease burden was also greater for the participants in the delayed treatment group compared with the early treatment group. Those in the delayed treatment group had both glaucomatous optic disc and visual field loss and had more glaucomatous structural and functional damage. The mean PSD slope of eyes that progressed to POAG was steeper in comparison to the early treatment group.
Treatment and management decisions should weigh the following factors: patient’s age at diagnosis, general health status, life expectancy and personal preference to treatment. For example, patients of older age with slowly progressing glaucoma likely require less intense treatment or sometimes no additional treatment at all. In comparison, young glaucoma patients with fast progressing disease require quick action, an aggressive approach and possibly surgery depending on the circumstances.13
 |
|
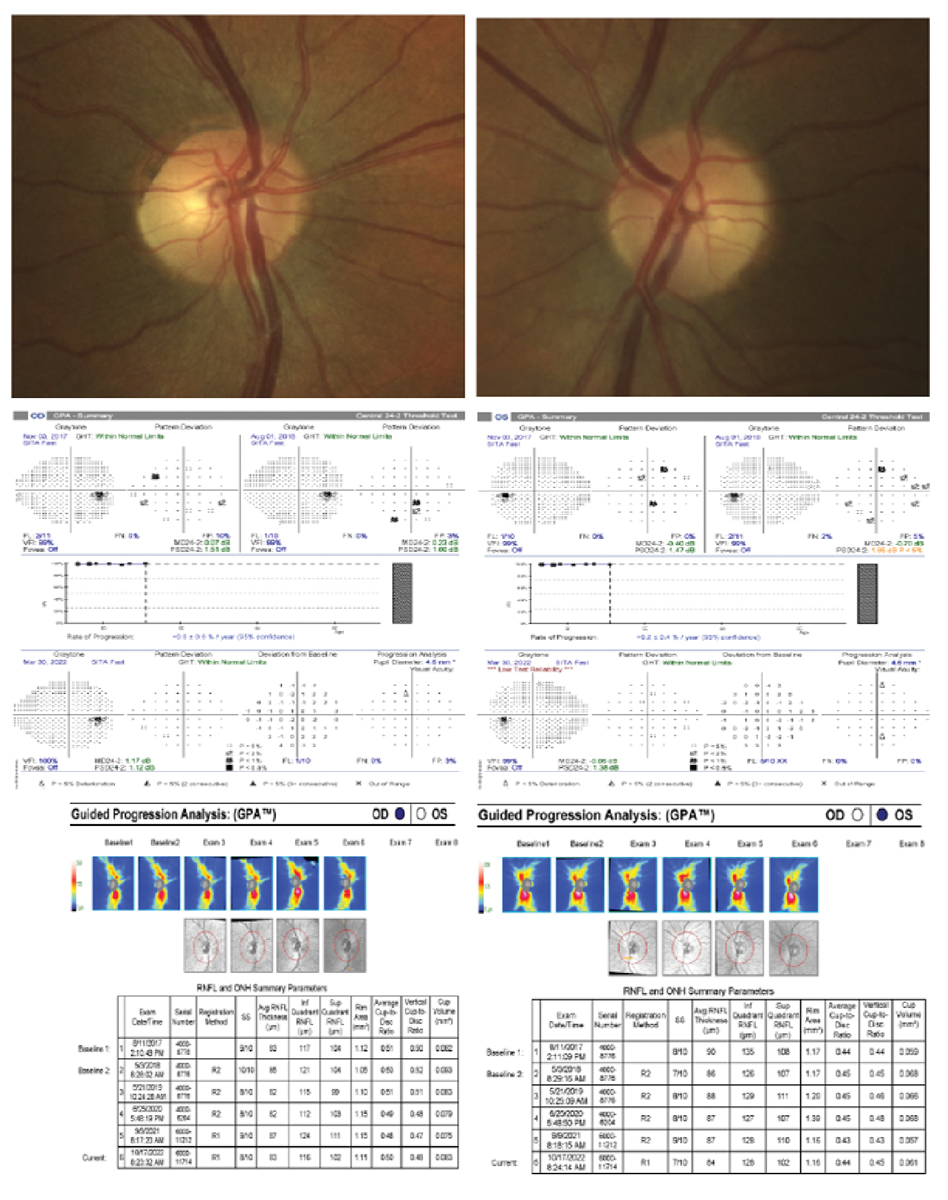
A 53-year-old white, non-Hispanic healthy male with OHT. His untreated IOP max was 27mm Hg OD and 28mm Hg OS, pachymetry measured 636µm OD and 640µm OS, VCDR was 0.35 OD and 0.40 OS and PSD was 1.12 OD and 1.38 OS. Based on these risk factors, no treatment was elected due to patient preference. His visual field and RNFL progression analyses have been stable for five years. We remain conservative with this patient due to his general health status and young age but still do not recommend treatment. He had no family history of glaucoma, no vessel barring or bayonetting, no notches or acquired pitting, no thinning of the rim, no disc hemorrhages, no localized RNFL dropout and changes in the depth of the cup. Click image to enlarge. |
OHTS-III
The final phase of the study is the 20-year observational follow-up to OHTS phases I and II.14 The OHTS Study Group has done an impressive job following a large sample of people over 20 years, collecting a wealth of information along the way to help guide clinicians in their decision-making for the treatment of OHT.
An extensive effort was made by the OHTS Study Group to follow-up with as many subjects as possible, resulting in long-term data for 971 (59.4%) of the original 1,636 participants in OHTS-I.15 Data on these subjects was collected from January 2016 to April 2019, or within two years of death, resulting in a median follow-up of 20.2 years. As of 2009 at the conclusion of OHTS-II, study participants were not treated under specific testing protocols. Treatment was left instead to the discretion of each subject’s eyecare provider. Of the OHTS-III subjects, 696 (72%) remained on OHT medications, and 296 subjects (30.5%) underwent some form of glaucoma surgery.
OHTS-III aimed to investigate the following objectives:
1. Determine the 20-year incidence and severity of POAG.
2. Determine the frequency and timeframe of POAG progression.
3. Develop a 20-year prediction model for stratifying OHT patients by their risk for developing POAG. The risks considered include both the original predictive factors from OHTS-I as well as newly identified risk factors.
4. Develop a prediction model for glaucomatous visual field loss rates.
5. Determine the frequency and severity of self-reported functional limitations associated with POAG.
Assessment of visual function over 20 years was done through OHTS examination, interim clinical data or medical records within two years of death. Classification of POAG in OHTS-III used the same methodology as the first two OHTS phases. Use of OCT was not part of the original two phases, but OCT measurements of the retinal nerve fiber layer (RNFL) and macula were obtained during data collection for OHTS-III. However, in maintaining consistency with the first two phases, OCT was not used in POAG diagnosis.
Of the original 1,636 subjects, 483 (29.5%) developed POAG in one or both eyes after 20 years. Upon further analysis, 199 participants (12.2%) had only optic disc deterioration in one or both eyes, 70 (4.3%) had visual field loss without disc deterioration in one or both eyes, 204 (12.5%) had both visual field loss and disc deterioration and 10 (0.6%) had visual field loss in one eye and disc deterioration in the fellow eye.
Throughout the 20-year course of the OHTS study, 665 (40.6%) participants were lost to follow-up or declined to participate in phase III. Another 515 participants (31.5%) died, naturally reducing their risk of developing glaucoma to zero. To account for this statistically, the conversion rate to POAG was also calculated with an adjustment for person-years of exposure time (totaling 21,864 person-years). This resulted in a 45.6% incidence of glaucoma at 20 years for all participants, or a 49.3% incidence for participants in the original observation group and a 41.9% incidence for participants in the original medication group.
When broken down by race, African American participants were statistically more likely to develop glaucoma at 55.2% compared with participants of other races at 42.7%; however, African American participants were also more likely to have a thin CCT and a larger VCDR. As mentioned previously, race was not statistically significant in OHTS-I after adjusting for thin CCT and large VCDR.10
Of considerable usefulness in OHTS-III is the cumulative incidence of POAG when adjusted for person-years of exposure time as divided into low-, medium- and high-risk groups. Subjects in the low-risk tertile had a 1.3% incidence of POAG at five years and a 31.7% incidence at 20 years. The medium-risk tertile had a 4.3% incidence of POAG at five years and a 47.6% incidence at 20 years, and the high-risk tertile had an 11.9% incidence of POAG at five years and a 59.8% incidence at 20 years. The 20-year cumulative incidence of visual field loss was found to be 25.2%.
 |
|
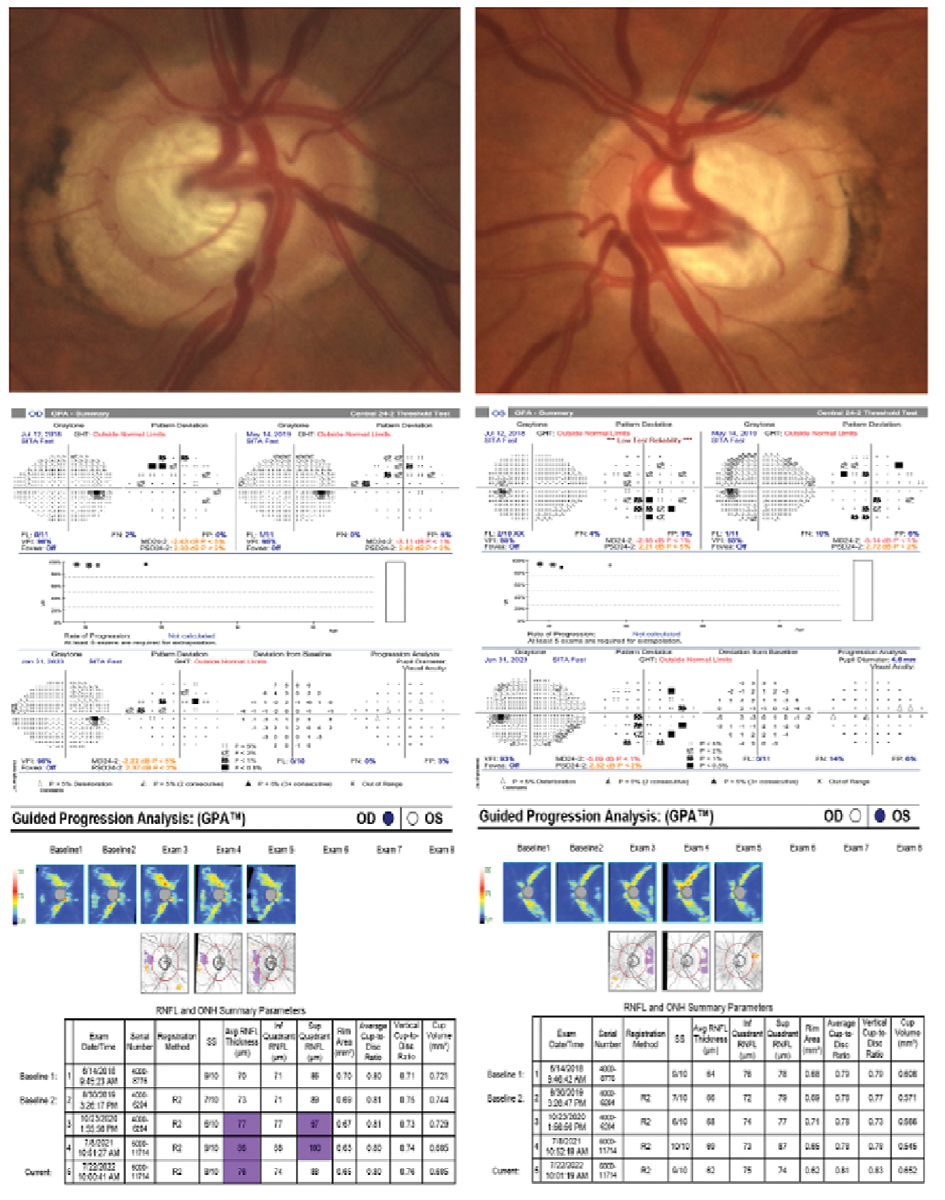
A 51-year-old white, non-Hispanic male with OHT, type 2 diabetes and a positive family history of blindness related to glaucoma. His untreated IOP max was 32mm Hg in both eyes, pachymetry measured 580µm OD and 582µm OS, VCDR was 0.80 OD and 0.85 OS and PSD was 2.37 OD and 2.52 OS. The extent of visual field and structural damage presumably occurred prior to establishing care at our clinic. He was immediately started on prostaglandin analog treatment and underwent cataract surgery due to a diabetic cataract. He had endoscopic cyclophotocoagulation and an iStent placed in both eyes. His post-surgical and medicated IOPs were lowered by 50%. His visual field and RNFL progression analyses have remained stable for the past five years. Early and aggressive intervention in this case was effective in preserving structure and function. Click image to enlarge. |
Treatment of OHT Patients
When you look at a 45.6% overall adjusted incidence of glaucoma at 20 years and the results from OHTS-I showing early topical medical therapy can reduce the risk of glaucoma by almost 60%, it seems straightforward to think all patients with OHT would benefit from treatment. OHTS phases I through III work together to demonstrate that while treatment is in fact effective at delaying or preventing POAG onset, medication is not something that is immediately necessary for all patients with OHT. This provides the clinician with evidence-based support on clinical decision-making. Low-risk patients can be followed less conservatively, with longer intervals of follow-up and delayed treatment, while medium- to high-risk patients should be followed at shorter intervals and started on treatment earlier.
While data from OHTS is very useful in everyday practice, it is also important to understand its limitations. The aforementioned risk predictions and incidences of glaucoma should only be applied to patients who meet similar characteristics of the OHTS cohort. For example, this data applies to generally healthy patients with IOP between 24mm Hg and 32mm Hg but not necessarily to patients suspected of normal-tension glaucoma, previous intraocular surgery, a life-threatening or debilitating disease, secondary causes of elevated IOP, angle-closure glaucoma or anatomically narrow angles, diabetic retinopathy and/or congenital or acquired optic disc abnormalities that can produce visual field loss.
The overall 20-year incidence of glaucoma may be higher in the OHTS cohort than in the general population for several reasons. The study intentionally over-represented African Americans at 25%, which may have biased the calculated higher incidence of glaucoma as compared with other races. Additionally, goals of IOP reduction of 20% or less than 24mm Hg were set in OHTS-I, and this may not have been adequate for all subjects.
Conversely, one could argue that the incidence of glaucoma may have been under-represented in the OHTS trial due to advances in diagnostic testing. OCT is now used to determine optic disc deterioration in combination with disc photos, rather than disc photos alone. This allows for earlier detection and diagnosis of glaucoma and could mean OHTS trial patients who were considered to have OHT based on enrollment criteria likely would have had detectable early POAG if OCT methods had been available at the origination of OHTS-I.
It has been shown that RNFL loss occurs before visual field defects, so it is surprising that OHTS-III found that 4.3% of subjects developed visual field defects without optic disc damage.16 It is possible that optic disc photos were not as sensitive in detecting early structural damage. This evidence illustrates the importance of using a combination of OCT, photos and visual fields to maximize sensitivity in detecting both structural and functional damage.
Even though the OHTS data has limitations, it can still serve as an excellent general guide when deciding whether to treat OHT. In a patient newly diagnosed with OHT, consider if they would fall under the low-, medium- or high-risk tertile based on the five baseline risks detailed earlier. While treatment in OHTS showed a 60% reduced risk of developing glaucoma, it is important to consider that the overall conversion rate to POAG (<10%) was still relatively low at five years, and this percentage would be even lower for someone categorized as low risk. There is an obvious burden to treatment in terms of cost, inconvenience and side effects. If a patient is considered lower risk, knowing that their risk of developing POAG in five years is approximately 1.3%, it would be reasonable just to observe without treatment and extend follow-up visits out longer than you would for someone in a higher risk category. This is also supported by data from OHTS-II which demonstrated that when comparing early treatment and delayed treatment groups, there was little difference in the cumulative number of patients who developed glaucoma.17
The clinical implications to initiate glaucoma treatment should incorporate an evidence-based approach from the results of these studies and include an assessment of the risk profile for each patient. The decision should consider the risks for functional vision impairment and decreased vision-related quality of life, ocular and systemic comorbidities, life expectancy, general health status and patient reservation about treatment.18 Considering that by year 20 of the OHTS trial there was an overall adjusted incidence of POAG at 45.6% and only 25.2% had visual field loss, we can conclude that initiation of treatment should be reserved for medium- to high-risk patients. This study offers reassurance that it is reasonable to monitor without treatment in the earlier years of OHT diagnosis. Treatment is indicated when the risks of progressive disease outweigh the risks and potential side effects of therapy.
Another major consideration is the rate of progressive disease. The rate of decline in progressing OHT and POAG patients can be highly variable.4,19,20 Some may progress slowly over the course of many years and decades, whereas others with aggressive disease may progress rapidly and eventually suffer from blindness or substantial impairment unless immediate interventions occur. Thus, evaluation in rates of change is a critical and fundamental factor in management of OHT and POAG, and treatment is generally indicated when such loss has been determined to be at a progressive, measurable rate. Optic disc changes and/or localized RNFL defects can predict functional loss in glaucoma. Those with detected structural loss and progressive damage should generally be treated.
Other specific risk factors to consider include baseline untreated IOPs, age, family history, worse disease severity at the time of diagnosis, optic disc hemorrhages, thinner corneas and pseudoexfoliation. Though OHTS phases I through III have shown progress in the identification of risk factors, more needs to be done to refine risk progression models. All these factors can affect the risk of progression and therefore help us pinpoint the expected prognosis of the patient’s untreated disease. Frequency of follow-up and aggressiveness of therapy can then be decided.4
Dr. Fisher is a staff optometrist and supervisor at the Villages VA Outpatient Clinic in the Villages, FL. He graduated from Midwestern University Arizona College of Optometry and completed his residency in primary care and low vision at the Lake City VA Medical Center in Lake City, FL.
Dr. Menjivar is a staff optometrist at the Ocala VA Clinic in Ocala, FL. She graduated from the Ohio State University College of Optometry and completed her residency in primary care and low vision at the Lake City VA Medical Center. Dr. Howard is a staff optometrist and supervisor for the Lake City VAMC and Gainesville VA Optometry clinics in north Florida. She graduated from the University of the Incarnate Word Rosenberg School of Optometry and completed her residency in primary care and low vision at the Lake City VA Medical Center.
Dr. Nguyen is a staff optometrist at the Phoenix VA Healthcare System in Phoenix, AZ. She graduated from Midwestern University Arizona College of Optometry and completed her residency at the Malcom Randall VAMC in Gainesville, FL.
Dr. Banister is a staff optometrist at the Villages VA Outpatient Clinic. She graduated from Pennsylvania College of Optometry at Salus University and completed her residency at the Bay Pines VA Healthcare Center in Bay Pines, FL. They have no relevant financial interests to disclose.
1. Tham YC, Li X, Wong TY, et al. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-90. 2. Sowka, JW. When to Treat Ocular Hypertension. Review of Optometry. 2020; https://www.reviewofoptometry.com/article/when-to-treat-ocular-hypertension. 3. Weinreb RN, D.F. Garway-Heath DF, Leung C, et al. 10th Consensus Meeting: Diagnosis of Primary Open Angle Glaucoma. Seattle, USA, April 30, 2016. 4. Weinreb RN, Makoto Araie M, Remo Susanna R, et al. 7th Consensus Meeting: Medical Treatment of Glaucoma. Fort Lauderdale, FL, May 1, 2010. 5. Gordon MO, Kass MA, for the Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: Design and Baseline Description of the Participants. Arch Ophthalmol. 1999;117:573-83. 6. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study – A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-13. 7. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20, discussion 829-30. 8. Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366-75. 9. Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123(10):1351-60. 10. Gordon MO, Beisesr JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20. 11. Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122(6):813-20. 12. Gordon, MO, Kass MA. What we have learned from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2018; doi:10.1016/j.ajo.2018.02.016. 13. Fisher B, Johnson D, Fisher A, et al. Glaucoma: Six Perils of Progression. Review of Optometry. 2020;157(7):40-5. 14. Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Study Group: Assessment of cumulative incidence and severity of primary open-angle glaucoma among participants in the Ocular Hypertension Treatment Study after 20 years of. Follow-up. JAMA Ophthalmol. 2021;139(5):1-9. 15. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study – A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-13. 16. Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741-8. 17. Kass MA, Gordon MO, Gao F, et al. Delaying Treatment of Ocular Hypertension: The Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128(3):276-87. 18. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363:1711-20. 19. Anderson DR, Drance SM, Schulzer M. Natural history of normal-tension glaucoma. Ophthalmology 2001;108:247-53. 20. Medeiros FA, Zangwill LM, Alencar LM, et al. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149:908-15. |
