Positive Visual Phenomena: Etiologies Beyond the Eye
Prepare to investigate the many non-ocular events that cause patients to see flashes or bright lights.
By
Release Date: January 2018
Expiration Date: January 15, 2021
Goal Statement: Often, clinicians assume a patient’s complaint of bright lights or flashes in vision are associated with vitreous detachment, retinal break or retinal detachment. However, it is critical that ODs carefully differentiate the source of these symptoms, as positive visual phenomena may indicate serious—even life-threatening—systemic health concerns. This article discusses how to identify less common—but higher risk—etiologies of positive visual phenomena.
Faculty/Editorial Board: Sara Weidmayer, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 55957-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian and Michael Iannucci all have no relationships to disclose.
We are all familiar with positive visual phenomena and photopsias. They are generally an entopic concern: visual perceptions produced from inside the eye, from vitreous traction on the retina, for example.1,2 However, other positive visual phenomena represent false visual images—the brain perceives them without corresponding visual stimuli.
When a patient reports any sort of bright light or flash in their vision, our first thought is often vitreous detachment, retinal break or retinal detachment. During a normal dilated eye exam, it is easy to attribute such symptoms to a migraine aura without headache (MAWH). However, the etiology of positive visual phenomena is often not intraocular, MAWH or even other benign causes. It is critical that we carefully differentiate the source of these symptoms, as positive visual phenomena may indicate serious—even life-threatening—systemic health concerns.
A key feature to differentiate the source of a flash is laterality; a unilateral flash generally corresponds to an ocular etiology, whereas bilateral flashes are more likely at, or posterior to, the chiasm.2 Because clinicians are already well-versed in ocular sources of flashes, this article discusses them only briefly, focusing instead on less common—but higher risk—etiologies of positive visual phenomena.
 |
| Migraine aura often includes a scintillating, or fortification, scotoma. Often, the central scotoma is bordered by a crescent of shimmering zigzags. Photo: George T. Banyas, OD |
Ocular Sources
Many flashes can be attributed to retinal pathology, such as posterior vitreous detachment, any vitreoretinal traction or a retinal break. Any mechanical stimulation—such as tugging or compression—of the photoreceptors can trigger an entopic flash in vision.1 In these cases, the flashes are often accompanied by floaters, such a Weiss ring, red blood cells or pigmented cells within the vitreous (“Shafer’s sign”) and associated fundus evaluation findings. Compression from mass effect within or onto the globe may occur from a variety of intraocular or orbital sources, ranging from choroidal lesions to orbital abnormalities such as tumors.
A thorough dilated examination should easily differentiate many of these sources of photopsia, and any signs indicating a retro-ocular compressive lesion such as proptosis, extraocular muscle motility restriction or diplopia would warrant further neuroimaging.
Photoreceptor dysfunction from inflammation or infections can also produce flashes in vision. Diseases such as progressive outer retinal necrosis, acute zonal occult outer retinopathy, retinitis pigmentosa, degenerative retinopathies or other issues that lead to photoreceptor death or dysfunction may produce a photopsia.1 Unlike many other ocular sources of flashes, these diseases most frequently affect both eyes, though they can be unilateral or asymmetric.
Retinal vasospasm causes a focal narrowing of the retinal arteriolar lumen, effectively limiting blood flow and possibly producing a visual phenomenon in the area of relative ischemia. Although this generally would produce a negative visual phenomenon, in some cases it may be accompanied by flashing lights. Retinal vasospasms are temporary and usually recover within a few minutes.3,4 In cases of unrecovered vasospasm, an associated visual field defect would be expected, as would an area of hypoperfusion on fluorescein angiography (FA) similar to a branch retinal artery occlusion. This diagnosis is rare and should be one of exclusion from more serious conditions such as transient ischemic attack (TIA) or cerebrovascular accident (CVA, stroke) and should be evaluated for such immediately.
Flashes or streaks of light can occur due to the positioning of an intraocular lens—known as pseudophakic dysphotopsia.5 These often present as arcs of light in the superior temporal periphery and can be quite bothersome to patients, but are otherwise benign.
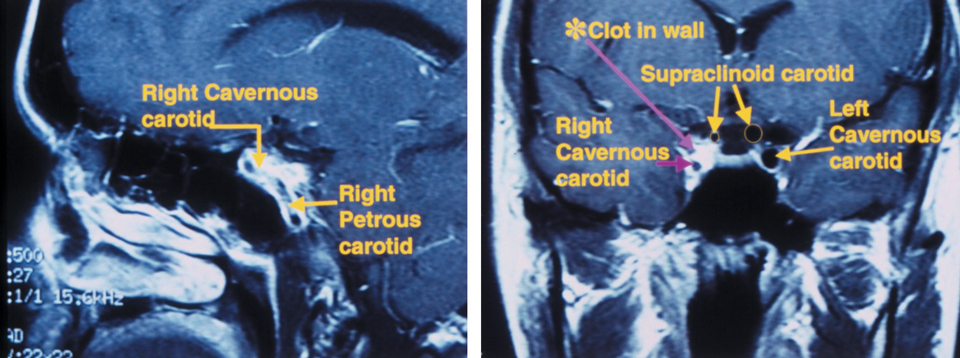 |
| This 36-year-old patient was complaining of constant periorbital headaches on the right side, accompanied by ptosis and miotic pupil. Four weeks earlier, she developed a temporal scintillating scotoma in her right eye that lasted 15 to 30 minutes, followed by an acute, painful headache on the right side. Sagittal MRI (left) shows an internal carotid artery dissection in the wall of the petrous and cavernous sinus segments. Coronal MRI (right) shows significant narrowing of the carotid lumen in the cavernous sinus and supraclinoid segments of the right internal carotid artery. The patient was diagnosed with a post-ganglionic right Horner’s syndrome with concurrent headache and scintillating scotomas. Photos: Ellen Marie Petrilla and Gina G. Wong, OD |
Non-ocular Etiologies
Generally, non-ocular causes of positive visual phenomena are binocular and originate from vascular- or non-vascular changes involving the cerebral cortex.
Migraine aura. This represents focal neurological symptoms that are reversible and may be seen in up to about 30% of patients with migraine.6,7 It also accounts for most cases of bilateral positive visual phenomena.6,8 In migraine sufferers with aura symptoms, 90% have visual symptoms.6,8 Visual migraine aura often follows a predictable pattern: it precedes or accompanies a headache and may also present with symptoms typical of migraine, such as nausea, photophobia and phonophobia, sometimes with focal weakness, numbness or paresthesia, dizziness or dysphasia.6,7,9 While migraine with typical aura is a straightforward clinical diagnosis, aura symptoms in the absence of headache is more difficult to discriminate from more serious etiologies such as TIA. Migraine aura is rarely a stand-alone migraine variant; this entity is known, among other names, as acephalgic migraine, optical migraine, migraine accompaniments, migraine equivalents or typical MAWH.6,10
Migraine aura normally starts gradually with a generally binocular, typical scintillating scotoma that subsequently intensifies over the course of five minutes to one hour, though 15% to 30% may extend beyond one hour.6,7,11 Many patients describe its onset as a small central flashing light expanding radially to include an enlarging area of visual field; it is often arcuate, or in many cases forms a shimmering or flickering jagged circular pattern, often called teichopsia, or described as a fortification spectrum.6,12 It usually also includes areas of negative visual features such as scotomas or hemianopia, a heat wave sensation and visual blur.6,7,10 When the aura is associated with migraine, it is most often contralateral to the headache, but can be ipsilateral.8 While many variations exist, a key component is the scintillating scotoma’s dynamic, traveling presentation.
Typically, after visual aura comes a speech aura in about one-fourth of patients, followed by a sensory aura in about one-third of patients. While this order of aura is typical, it may vary in about 30% of patients. Motor involvement describes hemiplegic migraine. Progressing through sequential aura is consistent with the cortical spreading depression from brief cortical hyperexcitability, which is thought to cause MAWH and may be helpful in diagnosing MAWH.13-15
In cases where patients present with visual symptoms similar to a migraine aura, especially with any associated neurological deficits and particularly in older patients or those with cardiovascular risk factors but no history of migraine or similar previous episodes of migraine or aura, clinicians must conduct a careful case history to rule out TIA, CVA, seizure, inflammatory cerebrovascular disease and vertebrobasilar insufficiency—all of which can cause positive visual phenomena.6,10
The current criteria for diagnosing MAWH require at least two episodes of aura. Therefore, any inaugural episode should first be treated as a TIA, as stroke risk is highest within two days of TIA—not to mention other causes are often uncovered in a TIA/stroke workup.7 Additionally, any aura that is less than five minutes or longer than an hour should be further evaluated for other causes.7,16
Of note, migraine is an independent risk factor for ischemic stroke, or migrainous infarction.7,10,12 However, transient visual symptoms, similar to those of MAWH, are not uncommon—even later in life—and do not appear to be associated with an absolute higher stroke risk.10
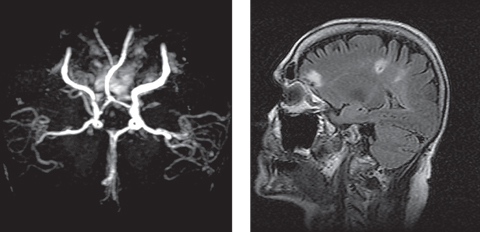 |
| This patient presented emergently with a complaint of three isolated instances of “blue-colored shadows” that transiently and incompletely blocked the vision of the right eye for five minutes at a time. At left, her MRA revealed severe stenosis of the right internal carotid artery (ICA), and she was diagnosed with amaurosis fugax and right hemispheric subacute CVA secondary to severe right ICA stenosis. At right, imaging also showed two restricted diffusion foci located within the right posterior parietal-occipital junction, consistent with a subacute infarct. Click photos to enlarge. Photos: Michael Trottini, OD, and Michael DelGiodice, OD |
Transient ischemic attack. TIA symptoms result from local ischemia, usually due to thrombotic or embolic vascular disease, but the TIA itself does not result in acute or permanent infarction.11,17 TIA affects about five million people per year in the United States.17 Ischemic stroke, on the other hand, indicates cerebral or central nervous system infarction and may either share similar symptoms with TIA or may happen without symptoms.17
The clinical symptoms of TIA are generally brief (a few seconds to a few minutes), and by definition there must be no infarct of the central nervous system.7 Common symptoms include numbness, weakness, tingling or paralysis of one side of the face or body; transient monocular vision loss (amaurosis fugax); aphasia or dysphasia; and dizziness.18 The visual symptoms are predominantly negative, present in corresponding hemianopic fields if the affected area is chiasmal or post-chiasmal, tend to be abrupt and at maximum within a couple minutes of onset and are stationary, as opposed to the gradual and dynamic visual symptoms with migraine aura.1,12,13 Though positive visual phenomena are less common, they can occur within an area of scotoma.12,13,19 As with non-visual symptoms, visual manifestations usually last less than five minutes, but may last up to 24 hours.
Patients who have experienced TIA symptoms should immediately undergo neuroimaging to evaluate for CVA, preferably MRI with diffusion-weighted imaging (DWI), within 24 hours of when the symptoms began, if possible; however, acute infarcts may initially appear normal on MRI because tissue injury has not yet become radiologically significant, and some deep brain infarcts cannot be well visualized with MRI imaging. Regardless, MRI is more sensitive than CT for identifying ischemic areas, and DWI-MRI studies are even more precise than standard MRI or CT.17 Positive DWI signals imply higher risk of subsequent ischemic events. If MRI is unavailable or contraindicated, clinicians should order a CT.17 An assessment of the intracranial arteries is often done concomitantly (with MR or CT angiography).20 Neuroimaging is important to identify or rule out a vascular (hypoperfusion, acute infarction, or large-vessel stenosis) or non-vascular (e.g., mass, abscess) origin of the TIA symptom.17
Clinicians should also evaluate the carotid arteries via noninvasive testing, such as Doppler ultrasound, as carotid stenosis is common in patients with atherothrombotic TIA or CVA. Basic TIA/CVA lab tests include complete blood count (CBC), blood chemistry panel and a basic coagulation assessment (prothrombin time, partial thromboplastin time).17
 |
| Image: Michael N. Block, OD |
There is no standard for cardiac evaluation in TIA patients, but the heart can be the underlying cause of TIA and accounts for 14% to 30% of ischemic CVA; for example, atrial fibrillation and recent myocardial infarction can lead to thromboemboli, and valve stenosis can lead to calcific emboli.21 Electrocardiograpy (ECG/EKG), cardiac event monitoring and echocardiography may be assessed if the initial evaluation did not uncover a source of the TIA symptoms; however, few patients with either no history of heart disease or with normal ECG will have a cardioembolic source for TIA.17
Seizures. These have a number of causes, including developmental malformations, ischemia or infarction, compressive lesions or trauma; they may also be idiopathic.13
While epilepsy, or recurrent seizures, usually presents with obvious seizure manifestations, seizure without convulsions are possible.13 In addition to motor, sensory and cognitive symptoms, seizure may be associated with nausea, headache and both positive and negative visual symptoms; however, it is uncommon for seizure to present with visual symptoms alone.13,22 Subtle seizures in epilepsy may present with oculomotor signs such as nystagmus, abnormal repetitive blinking or eyelid flutter and tonic alignment deviations.13
Visual symptoms associated with seizure are quite diverse; they may appear as flashes, patterns or colors in the vision. Patterns can change and may look similar to a fortification pattern. Complex visual hallucinations, including perception of animals or people, may occur, as can image enlargement (macropsia) or reduction (micropsia), to name a few.
The visual phenomena may be stationary, traveling, localized or encompassing the entire visual field. Negative visual symptoms such as scotomas, ranging from focal to hemifield to complete visual loss, may also occur, though negative symptoms are less frequent than positive visual symptoms.13,22
Focal occipital seizure most frequently causes these visual phenomena, particularly the positive symptoms. Visual symptoms with seizure tend to be brief, lasting only a few seconds, and may recur throughout the day.13 If seizure is suspected, electroencephalogram (EEG) may help detect an epileptic wave of activity, though it may be difficult to detect in cases where only a small area of cortex is involved.6,22
Other visual hallucinations. Visual hallucinations may occur as a result of psychiatric disorders; alcohol intoxication or withdrawal; and LSD, marijuana, mescaline, digoxin or other drug use.12,23 In these cases, the hallucination may be quite complex and may have accompanying auditory or other sensory hallucinations.12
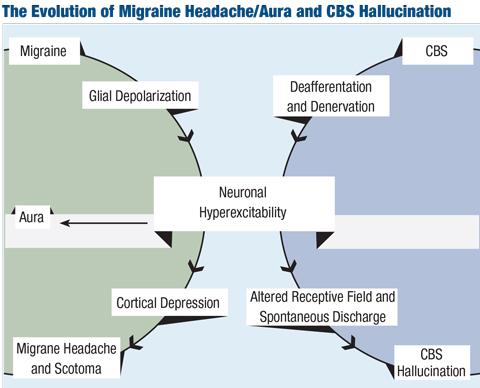
Charles Bonnet syndrome, also known as visual release phenomenon, may also produce simple or complex visual hallucinations. This condition is seen in patients who have acquired vision loss for any number of reasons, whereby the visual cortex produces the perception of these hallucinations, thought to be due to chronic lack of sensory input (deafferentation).24 Visual hallucinations in Charles Bonnet syndrome are not accompanied by any other sensory hallucinations.23
Positive visual phenomena, often a flickering sensation, may also occur due to systemic hypo- or hyperglycemia, or orthostatic hypotension.25 Researchers have reported formed and unformed visual hallucinations, particularly in hyperglycemia, which may precede or accompany other systemic symptoms of hypeglycemia such as change in mentation.26 These visual phenomena should subside with the normalization of systemic glucose or blood pressure.
| Table 1. Features Typically Associated with MAWH, TIA, CVA and Seizure | |||||
| History | Visual Symptoms | Onset | Duration | Neuroimaging/Ancillary Studies | |
| MAWH | History of migraine | Predominantly positive, dynamic | Gradual | Five to 60 minutes | Normal |
| TIA | Vasculopathic risks | Predominantly negative, static | Acute | Less than five minutes, typically; up to 24 hours | Likely ischemic cerebrovascular disease, no infarct |
| CVA | Vasculopathic risks | Predominantly negative, static | Acute | Less than five minutes | Area of cerebral infarct |
| Seizure | History of seizure or epilepsy, head trauma, vasculopathic risks | Predominantly positive, dynamic | Acute | Transient | EEG most useful |
Differentiating TIA, MAWH and Seizure
Visual symptoms are quite similar for seizure, MAWH, TIA and CVA. Migraines are frequently associated with epilepsy, as they share similar clinical features and pathophysiologic components; migraine and epilepsy may be concomitant, and the incidence of migraine in patients with epilepsy is about twice that of the general population.13,27 Because positive visual phenomena from seizure are brief and rarely stand-alone, any additional seizure-like symptoms would warrant an evaluation by neurology.
No specific features uniformly differentiate TIA from migrainous visual phenomena, and neurological work up is generally normal for both.7 Differentiating the two, however, is crucial, since TIA carries a 10% to 15% risk of subsequent stroke within three months, with half of those occurring within two days.7,17 The long-term risk of stroke and major cardiac events such as myocardial infarction also increases; thus, misdiagnosing a TIA as a migrainous aura could have serious—potentially life threatening—implications.7,17
While no universal differentiators exist, some frequent differences between MAWH and TIA can help (Table 1). First, history is important; often, patients with MAWH have had a history of migraine earlier in life. Patients with TIA usually have vasculopathic risk factors for cerebrovascular disease such as diabetes and hypertension.
In addition, MAWH presents with bright, glistening, dynamic visual aura that gradually progresses, while TIA generally shows a largely negative visual phenomenon with a flat, static, non-progressive presentation that is maximum at onset. On neuroimaging, MAWH often has normal results; TIA must not show infarction, but often shows evidence of cerebrovascular ischemia, such as chronic small-vessel changes, and additional vascular testing would likely show atherosclerosis.6
In cases of positive visual phenomenon or aura that are inaugural in patients older than 40, if visual symptoms are purely negative or if the duration of aura is atypical for MAWH, the episode must be treated as TIA until proven otherwise, and clinicians must promptly initiate a full TIA evaluation.16
Positive visual phenomena may occur for a number of reasons not easily detectable on a comprehensive eye exam. Clinicians often need to order ancillary studies and consult with other disciplines to arrive at the appropriate diagnosis, or to rule out pertinent differentials before attributing a more benign diagnosis of exclusion to a patient’s symptoms. Understanding the many potential underlying etiologies and other typical features of these conditions can help guide clinicians to the appropriate diagnosis and subsequent management.
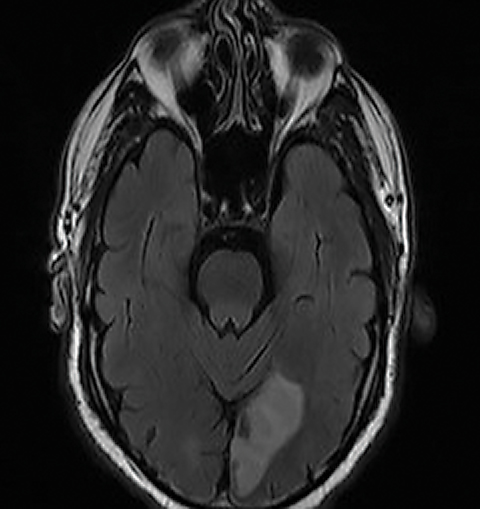
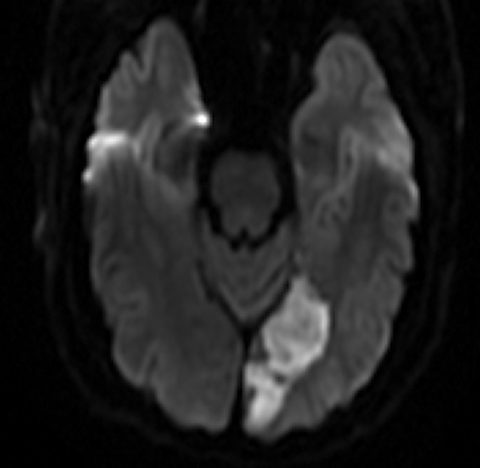
Case Report When questioned, he said he had a headache a couple days prior to the onset of the visual symptoms, and his ex-wife had recently asked if he had developed some speech problems, but he himself hadn’t noticed any changes. He denied tingling, weakness or numbness on either side of his face or body. During the exam, it became quite apparent that the patient was confused and forgetful, and he exhibited some labile emotional responses (such as spontaneously starting to cry when asked questions). The patient’s ocular health exam was largely unremarkable. Given that the patient was experiencing a visual phenomenon with both positive and negative features and visual hallucinations, along with subacute confusion, he was escorted to our hospital’s emergency department and was worked up for stroke. He was diagnosed with a left occipital lobe (left posterior cerebral artery territory) subacute infarct with restricted diffusion extending into the left temporo-occipital region, and ultimately was admitted (Figure 1). Days after admission, the patient began to notice visual loss in the bottom right portion of his vision, and the neurologist noted that the patient had developed a right homonymous inferior quadrantanopia. Neurology initially thought his positive visual phenomena represented either simple focal seizures or were a result of the infarct itself. An EEG indicated left hemispeheric slowing and moderate encephalopathy but no epileptiform discharges or seizures. His visual hallucinations and positive visual phenomena persisted even beyond discharge; they varied from seconds to 30 minutes and presented from every hour to five to six times per day and predominantly presented in the inferior right quadrant of his vision, where he’d developed the visual field defect; at subsequent follow up, neurology felt his visual hallucinations were most consistent with visual release phenomenon. As evidenced by this patient, some cases of positive visual phenomenon can be quite complex. This patient’s positive visual phenomenon was the first stroke symptom, and, as his eye care provider, I was well-positioned to take a thorough history and ensure he was appropriately referred. | ||||
Dr. Weidmayer is staff at the VA Ann Arbor Healthcare System in Ann Arbor, Mich., and a clinical assistant professor at the Kellogg Eye Center, Department of Ophthalmology and Visual Sciences at the University of Michigan.
1. Brown GC, Brown MM, Fischer DH. Photopsias: a key to diagnosis. Ophthalmol. 2015;122:2084-94. |