 |
Optic Nerve Disorders: How They Manifest and What They Mean
Understanding how to accurately identify, diagnose and manage these conditions
is a key component of optometric care.
By Marc D. Myers, OD, and Andrew S. Gurwood, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: July 15, 2023
Expiration Date: July 15, 2026
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists engaged in in optic nerve disorder management.
Educational Objectives: After completing this activity, participants should be better able to:
Accurately identify optic nerve disorders.
Distinguish between various differential diagnoses.
Perform the necessary diagnostic tests to properly diagnose these conditions.
Manage patients with optic nerve disorders.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Drs. Myers and Gurwood have nothing to disclose. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #126309 and course ID 85110-NO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
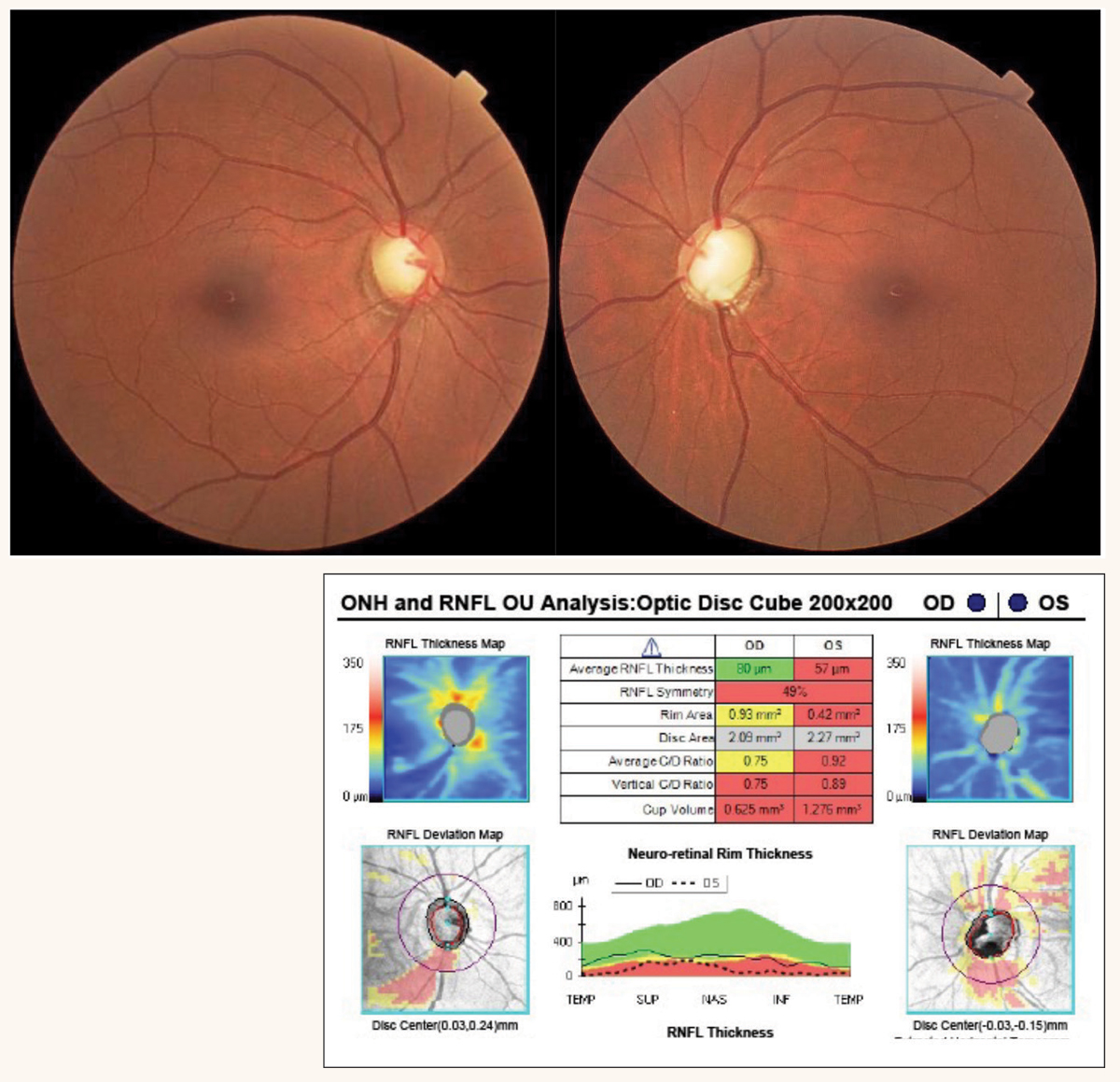 |
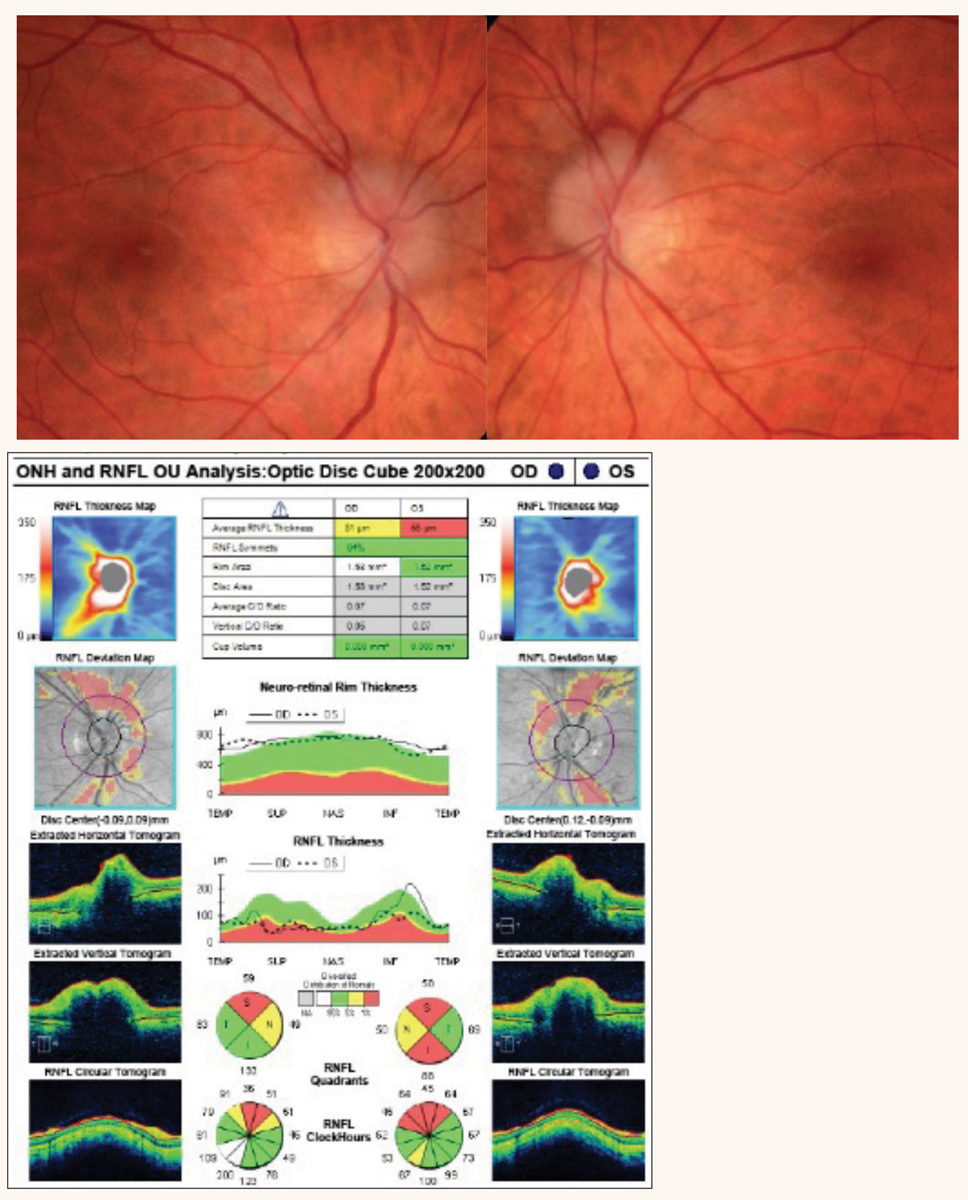
Fig. 1. Tilted discs with RNFL dropout OD and congenital optic pit OS. OCT reveals RNFL defects in the interior quadrant consistent with disc tilt. Click image to enlarge. |
There are a variety of disorders that can affect the optic nerve, and optometrists play a key role in the management of patients with these conditions. Effective treatment requires the ability to accurately identify optic nerve disorders and distinguish between the various diagnoses. This article will not only delve into how to recognize these conditions in clinical practice but will also discuss diagnostic tests and management approaches for comprehensive—and effective—patient care.
Congenital and Hereditary Optic Nerve Malformations
The optic nerve may be atypical and malformed with functional consequences at birth. Congenital optic nerve malformations are nonprogressive and have a broad spectrum of functional vision limitations.3-7 Early diagnosis should include ruling out any association with neurologic or systemic disease.
Congenital presentations, such as hypoplasia, tilted disc, optic pit and optic nerve head drusen, are common and frequently present with minor functional consequences (Figures 1 and 2).3,4 Coloboma, peripapillary staphyloma and morning glory syndrome can have a greater bearing on both the structure and function of the optic nerve. In addition, Aicardi syndrome (in which all or part of the corpus callosum is missing) and papillorenal syndrome (optic nerve dysplasia and renal hypoplasia) can occur with more significant visual and systemic consequences.3,4
Hereditary optic neuropathies are associated with progressive visual decline.4 Noteworthy are dominant optic atrophy, Leber’s hereditary optic neuropathy, optic atrophy with neurologic or systemic disease, Wolfram syndrome (juvenile-onset diabetes mellitus, optic nerve atrophy, hearing loss, neurodegeneration) and Costeff syndrome (optic nerve atrophy, delayed development, movement disorders). Vision loss in these cases is often profound and associated with neurologic and/or systemic disease.4
Acquired Optic Nerve Disorders
Beyond congenital and hereditary malformations, disease of the optic nerve can be the result of many etiologies. Glaucoma is a progressive optic neuropathy of a mixed mechanism. Non-glaucomatous optic nerve damage may be the result of compressive, toxic/nutritional, traumatic, vascular and inflammatory causes, as well as intracranial space-occupying lesions.
A Closer Look at the Optic NerveCranial nerve II, the optic nerve, is formed by the axons of approximately 1.2 million retinal ganglion cells.1,2 As the most anterior structure of the visual pathway, it contributes to the transmission of the electrical impulses from the retina to the brain. Disorders of the optic nerve often have devastating visual consequences and can be the result of congenital, hereditary or acquired diseases. Measuring approximately 6cm in length, the optic nerve is divided into four segments based on anatomical location.1,2 The intraocular section of the optic nerve consists of prelaminar and postlaminar sections, measuring approximately 1mm. The intraorbital, or postlaminar, section is approximately 3cm in length and courses from the globe to the apex of the orbit. The intraorbital segment is surrounded by orbital contents, including the recti muscles.1,2 The sheath of the superior and medial recti adhere to the sheath of the optic nerve, attributing to the pain associated with eye movements in a diagnosis such as ON.2 Continuous with the meningeal coverings of the brain, the dura, arachnoid and pia mater surround the intraorbital optic nerve. The outermost dura mater consists of dense connective tissue containing elastic fibers. The inner arachnoid layer is next to the dura. The subarachnoid space is continuous with the intracranial subarachnoid space and contains CSF. The pia mater sends blood vessels and connective tissue septa into the nerve.1,2 The intracanalicular portion of the optic nerve measures approximately 1cm and passes along the ophthalmic artery through the optic canal. The intracranial segment measures 1.6cm and travels within the suprasellar cistern. The right and left intracranial segments join to form the optic chiasm.1,2 The function of the optic nerve is purely sensory, as it contains only afferent fibers. Its functional capabilities include VA, perception of brightness, color and contrast, as well as both light and accommodation reflexes.1,2 |
Glaucoma
Glaucomatous optic neuropathy (GON) occurs in the setting of chronic, painless vision loss associated with variable optic atrophy.5-10 “Cupping,” a form of optic nerve atrophy, describes the enlargement of the cup-to-disc ratio. Optic nerve cupping in glaucoma is thought to result from the loss of ganglion cell axon fibers and the thinning and posterior displacement of the lamina cribrosa (Figure 3).8,9 Clinical characteristics of glaucoma include elevated intraocular pressure (IOP), cupping, spared central visual acuity (VA) and color vision (until late stages) and characteristic visual field defects (nasal step, arcuate pattern).
Treatment of GON is aimed at limiting the progression of damage to the optic nerve by reducing IOP by either medical or surgical means.8-10 Numerous efficacious topical and oral medications are available that target aqueous humor suppression, aqueous outflow through both the trabecular meshwork and uveoscleral pathway and reduced resistance from episcleral venous pressure. Surgical procedures, both laser and traditional, remain options in both early and advanced stages of glaucoma.8-10
In the setting of normal IOP and in early disease when structural and functional findings are not as obvious, consider other causes of optic nerve atrophy, including compressive optic neuropathy (CON), hereditary optic neuropathy and arteritic anterior ischemic optic neuropathy (AION).4-11,15,29
Compared with GON, patients with CON are often younger with worse VA and visual defects that may respect the vertical meridian. Also, pallor of the neuroretinal rim (vs. thinning) is more common in non-glaucomatous cupping.5-10 OCT provides structural information about the optic nerve head, RNFL and ganglion cell complex (GCC). OCT angiography assesses the peripapillary and macular microcirculation. Studies have revealed decreased retinal vessel density in CON.5,6
Non-Glaucomatous Causes
Accurate identification and diagnosis of optic nerve disorders requires the ability to distinguish between glaucomatous and non-glaucomatous optic neuropathies, which can be challenging. Next, we will discuss the various manifestations of these conditions and how to recognize and approach each.
Compressive Optic Neuropathies
These conditions are the result of space-occupying lesions that disrupt the physiology of both the optic nerve and retinal ganglion cells.11,15-17 Common neoplasias that may cause CON include glioma, meningioma, hemangioma, craniopharyngioma and pituitary adenoma (Figure 4).
Thyroid eye disease, aneurysms and ethmoid or sphenoid sinus mucocele are non-neoplastic causes of CON.15-17 Compression may occur along the course of the four segments of the nerve, resulting in unilateral or bilateral nerve involvement in the event of a chiasmal lesion.15-17
In a recent population-based study, compressive optic neuropathy was found to occur at an incidence of 1.14 per 100,000 people per year. The median age at diagnosis was 55, with 61% of patients being female.15
Pituitary adenoma was the most common cause of CON, accounting for 35% of all cases.15 Presenting symptoms included visual field loss, loss of color perception and decreased visual acuity. Diplopia may be the result of ocular motor nerve compression or mechanical restriction of the extraocular muscles due to orbital tumor or thyroid myopathy. Progression of symptoms is typically gradual but may be acute and dramatic in cases of intracranial aneurysm in close proximity to the optic nerve or in pituitary apoplexy.11,15-17
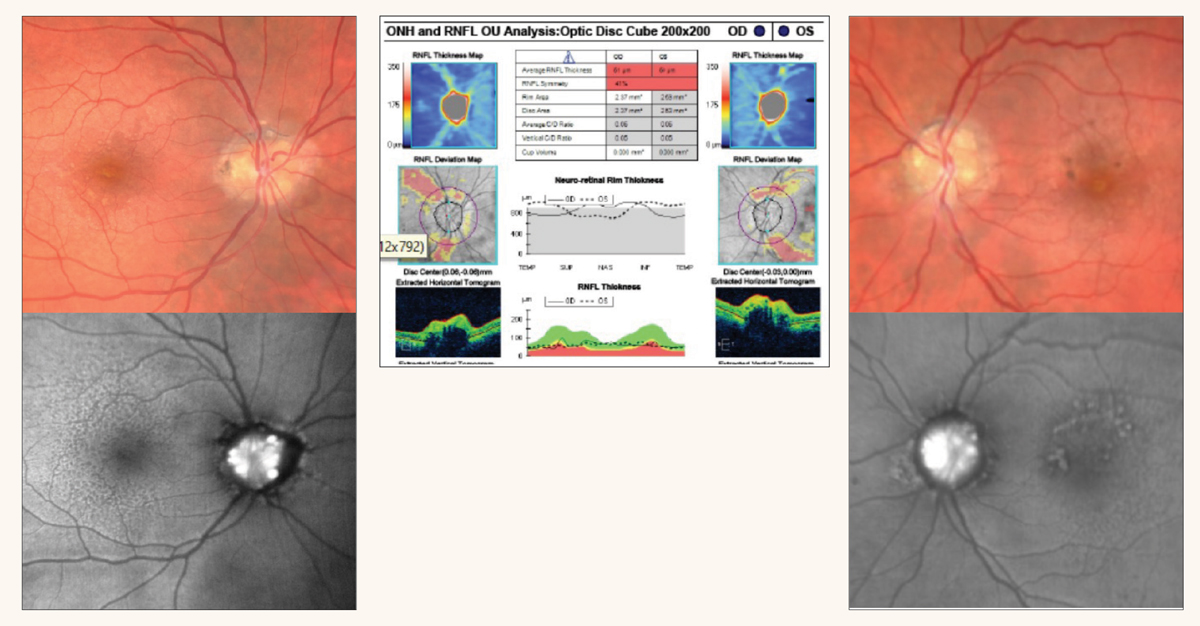 |
|
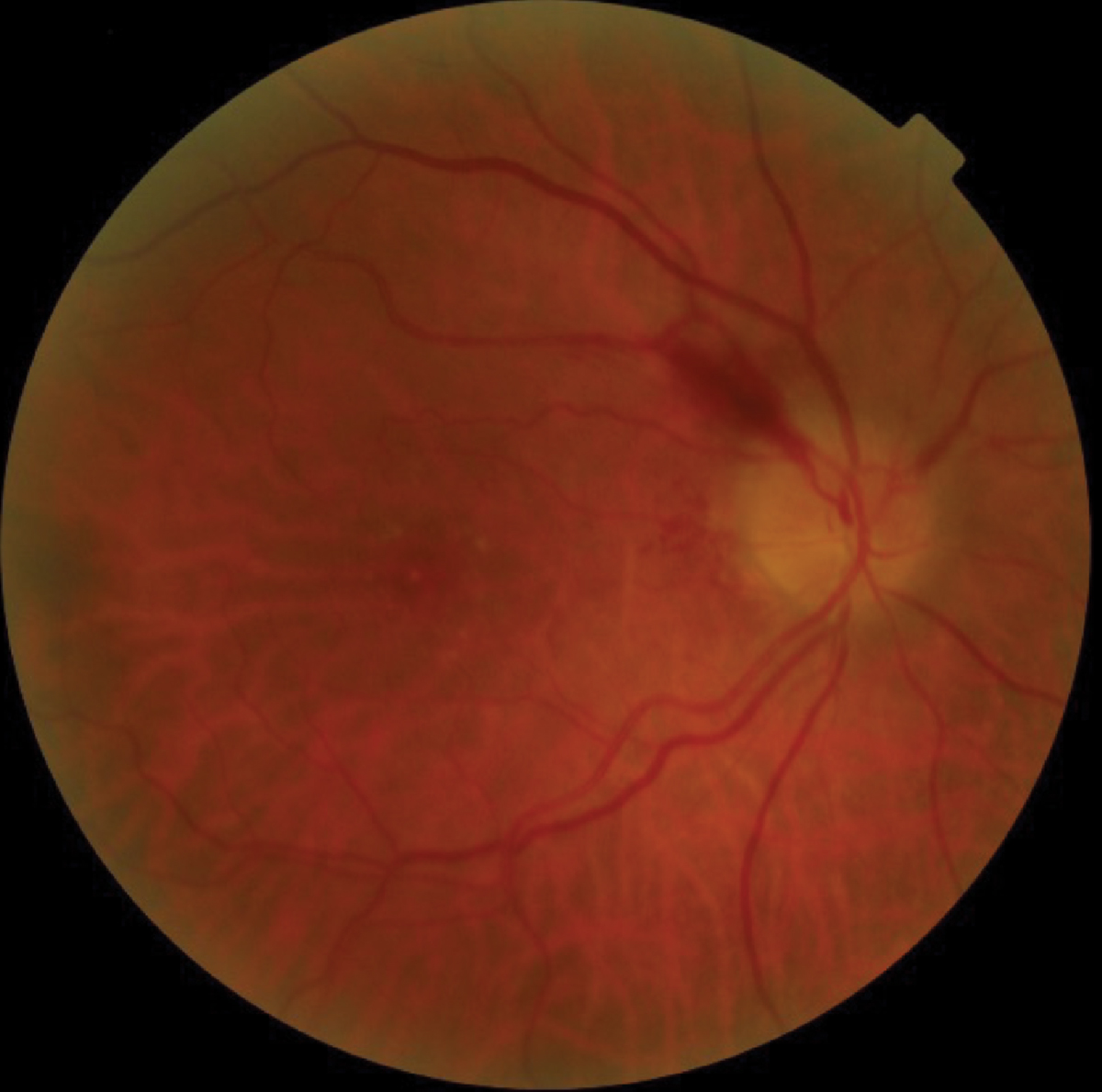
Fig. 2. Color and fundus autofluorescence images of optic disc drusen involving both the right and left eyes. OCT reveals RNFL thinning and elevated optic disc margins. Click image to enlarge. |
Clinical assessment is variable, as early cases may present with subtle signs of optic nerve impairment. Acuity may range from normal to acutely reduced. A relative afferent pupillary defect (RAPD) will be present in unilateral optic neuropathy. Color testing is also often impaired. Assessment of the optic disc may vary when presenting as normal, swollen, pale or cupped. Eye pain and headaches can be symptoms of CON.15-17
Visual field analysis in patients with pituitary adenoma will show bitemporal hemianopsia that is denser superiorly or a junctional scotoma. Craniopharyngiomas most typically present with bitemporal hemianopsia, denser inferiorly.15-17 Other causes of CON display a variety of patterns of visual field defects, such as cecocentral scotomas or generalized depression, depending on the location of the lesion.15-17 In cases of early detection and successful treatment, visual field defects may improve over time.
Inner retinal layers, including the RNFL, GCC and inner plexiform layer, are of particular interest in cases of neuro-ophthalmologic disease.14-17 The GCC is where the bodies of the ganglion cells are found. The RNFL is made up of axons of retinal ganglion cells that course out of the eye via the optic nerve, chiasm and tract, eventually synapsing in the lateral geniculate body.14
Compressive lesions may manifest as thinning of the RNFL and GCC because of the damage to retinal ganglion cell axons in the optic nerve, chiasm or tract. OCT of the RNFL and GCC is a method to quantify anatomic changes associated with pathology involving the anterior visual pathway.15 Thinning of the RNFL and GCC may proceed vision loss, allowing for earlier diagnosis of CON. Early diagnosis can be a key element in preserving structure(s), in this case the RNFL and GCC, and may help limit visual impairment.15-17
Signs of CON accompanied by impairment of ocular motility, decreased corneal sensitivity or ptosis serve as an indication of multiple cranial nerve involvement.15 Confirmation of a diagnosis involving a compressive lesion is made most frequently using MRI and CT.12,13,15
Management of compressive optic neuropathy ranges from observation to intracranial surgery. If a benign mass that is not threatening an adjacent structure proves to be stable in size and has no association with visual function, clinical observation with serial diagnostic testing may be recommended.15-17 Pharmacologic agents are used in the treatment of hormonally active pituitary tumors. Modern treatment plans have great success, as they include some combination of surgery (craniotomy, endoscopy), chemotherapy and radiation.15-17
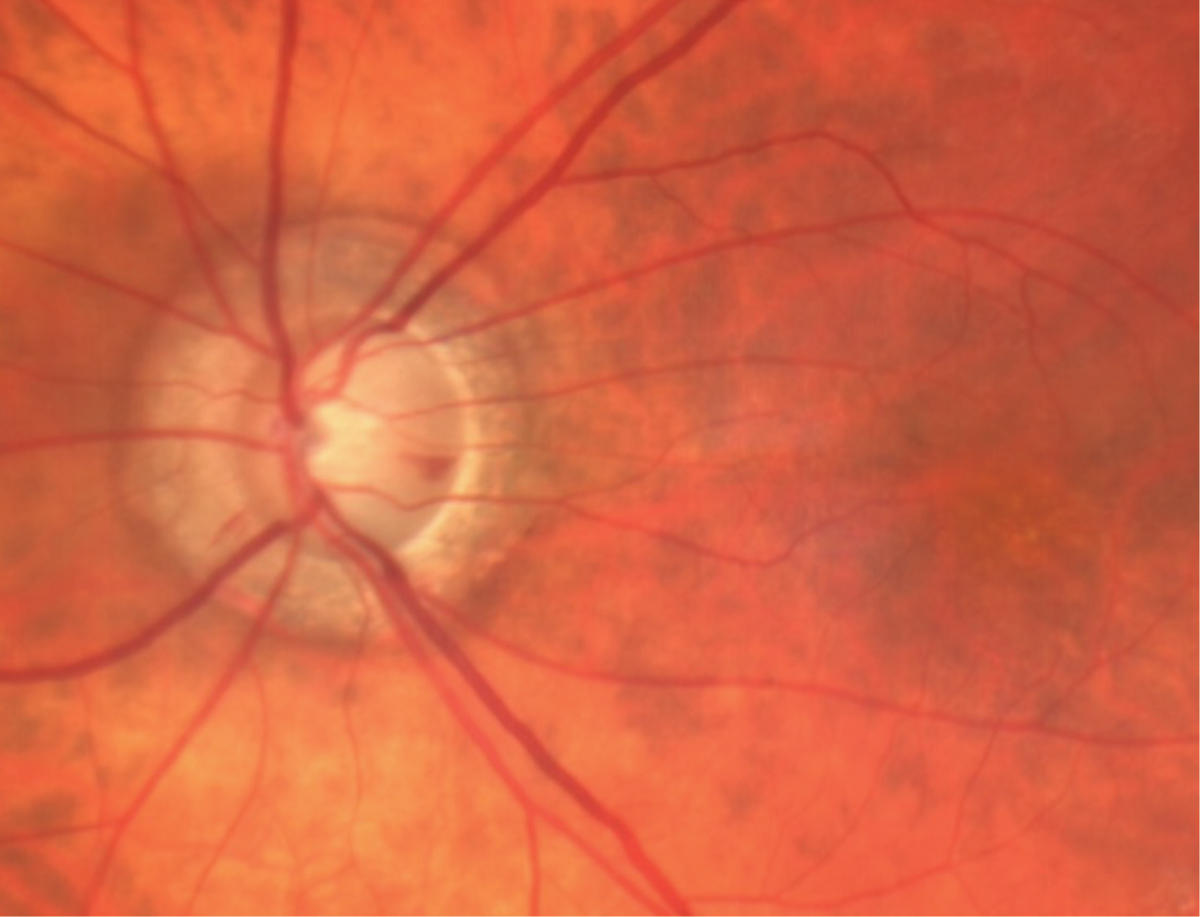 |
|
Fig. 3. Glaucomatous optic disc cupping, optic disc hemorrhage and atrophy surrounding the nerve. Click image to enlarge. |
Elevated Intracranial Pressure and Papilledema
Optic nerve disorders may be associated with elevated intracranial pressure (ICP).18-24 Papilledema refers to bilateral optic disc swelling secondary to elevated ICP (Figure 5). The most common cause of elevated ICP is idiopathic intracranial hypertension (IIH). As the name implies, in IIH there is no identifiable cause for elevated ICP.21-23
Other non-idiopathic etiologies that can cause either an increase in cerebrospinal fluid (CSF) production, decrease in CSF resorption and/or obstruction of CSF flow include space-occupying lesions, venous sinus thrombosis, diffuse cerebral edema, spinal cord masses, meningitis, malignant hypertension and toxic pharmacologic effects.11,18-24
Electron microscopy demonstrates that the optic disc edema of papilledema is primarily intra-axonal, influencing energy-dependent axoplasmic transport.11,19,20 It is the stasis of the intra-axonal fluid, swelling of axons and leakage of cellular contents into the extracellular space of the optic disc that gives rise to optic disc edema. Further, the reduced perfusion of axons may result in a secondary phenomenon of venous obstruction and dilation, nerve ischemia and vascular telangiectasia.11,19,20
Papilledema may occur in all ages, races and ethnic groups, as well as both genders. However, in papilledema specific to IIH, 90% of patients are female with an average age of 29, Caucasian predilection and average BMI of 39.9.19-21
In the setting of elevated ICP, regardless of the etiology, patients typically complain of headache, nausea, vomiting and visual symptoms, including blurred vision, transient visual obscurations (TVO), photopsias and diplopia.19-21 In the Idiopathic Intracranial Hypertension Treatment Trial, 84% of patients reported headache, with 68% describing features similar to migraine. The second most common complaint was TVO, occurring in 68% to 72% of patients. Other symptoms include pulsatile tinnitus, back pain, dizziness, photophobia, neck pain, vision loss and diplopia.21,22
Clinical examination will reveal optic disc edema that is bilateral and may be asymmetric. Bilateral or unilateral cranial nerve VI (abducens) palsy may also be present. In cases of IIH, the clinical examination must be otherwise normal.18-22 The Modified Dandy Criteria must be met in order to make the diagnosis of IIH (see sidebar). Beyond the identification of ophthalmic and systemic signs and symptoms, management criteria includes the assessment of CSF via lumbar puncture (evaluating both ICP and CSF cytology) and neuroimaging (completed prior to lumbar puncture) to rule out any potential underlying diagnosis that could cause increased ICP.24
If papilledema is the result of a mass, surgical resection may be indicated depending on the location. Anticoagulation and/or endovascular stenting is indicated to treat venous sinus thrombosis, a less common cause of papilledema. In cases of papilledema due to IIH, the severity of symptoms guides nonsurgical and surgical options.11,18-24
When visual changes have a gradual onset and are mild, a weight loss goal of between 5% to 10% of total body weight is associated with improvement of signs and symptoms.21-23 Acetazolamide combined with weight loss has proven effective in benefiting symptomatic patients. Topiramate reduces the symptoms of headache and aids in weight loss.21-23
When conservative management does not improve symptoms or makes them worse, surgical intervention is indicated. Optic nerve sheath fenestration involves surgically decompressing the optic nerve by creating a slit or window in the dura mater of the optic nerve, allowing the egress of the CSF from the subarachnoid space.22,23 Fenestration is used in cases of visual symptoms of an acute onset. It proves to be less effective in improving visual symptoms in cases where papilledema is chronic. Also, it is less effective at relieving headache symptoms when compared with CSF diversion procedures.22,23
If the predominant symptom associated with papilledema is headache, CSF diversion procedures such as ventriculoperitoneal shunt or lumbar peritoneal shunt are the more efficacious treatment options.22,23 If these interventions fail, endovascular venous sinus stenting (EVSS) can be considered when diagnostic imaging confirms stenosis of the transverse sinus. EVSS may result in symptomatic improvement in visual symptoms.22,23
When papilledema is caused by IIH, permanent vision loss is the most feared outcome.21,22 Hypertension is one of the greatest risk factors associated with a poor visual prognosis. Other contributors to poor prognosis include rapid symptom onset, progression, the severity of the presenting visual symptoms and the rate at which those symptoms deteriorate.11,21-24
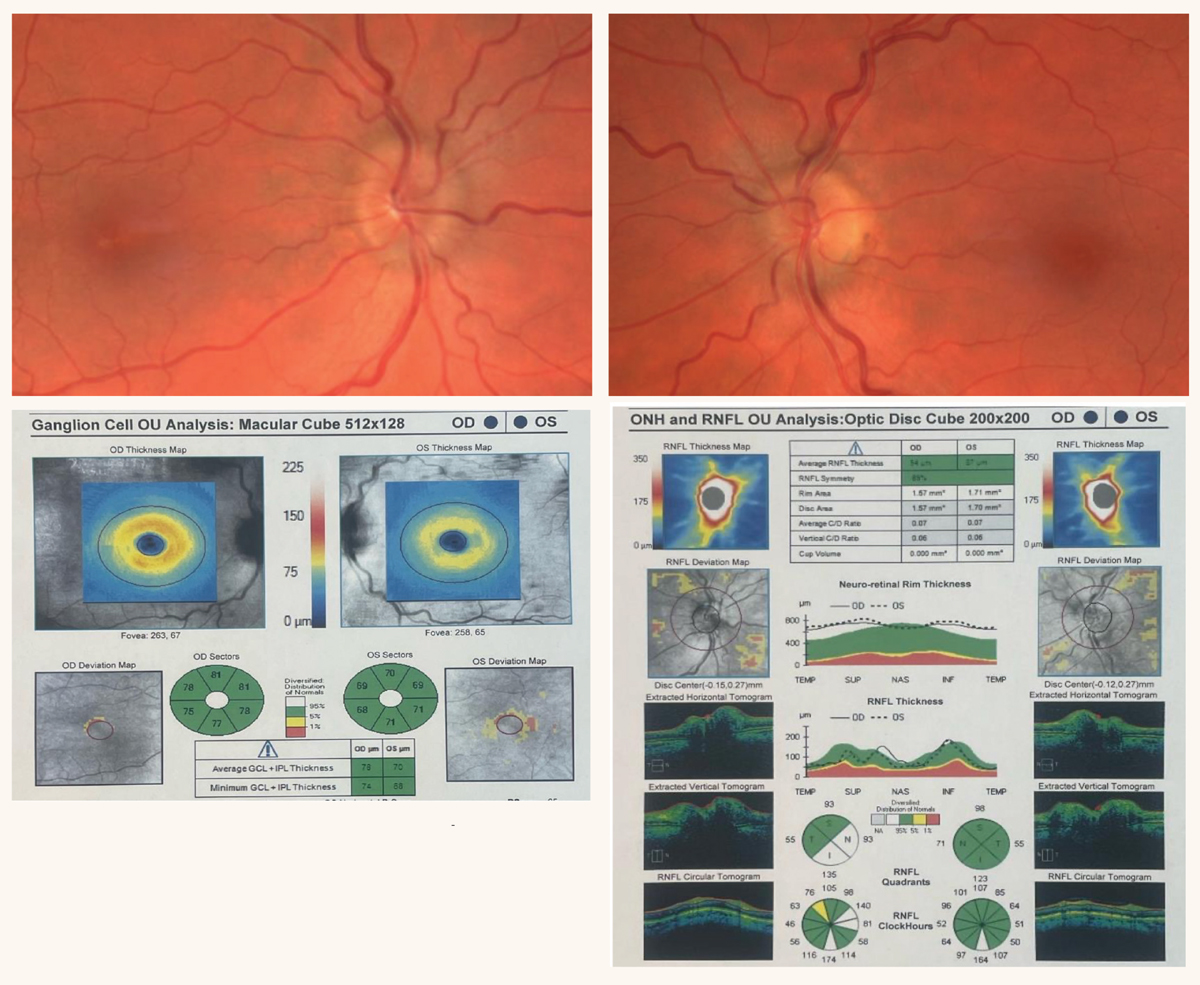 |
|
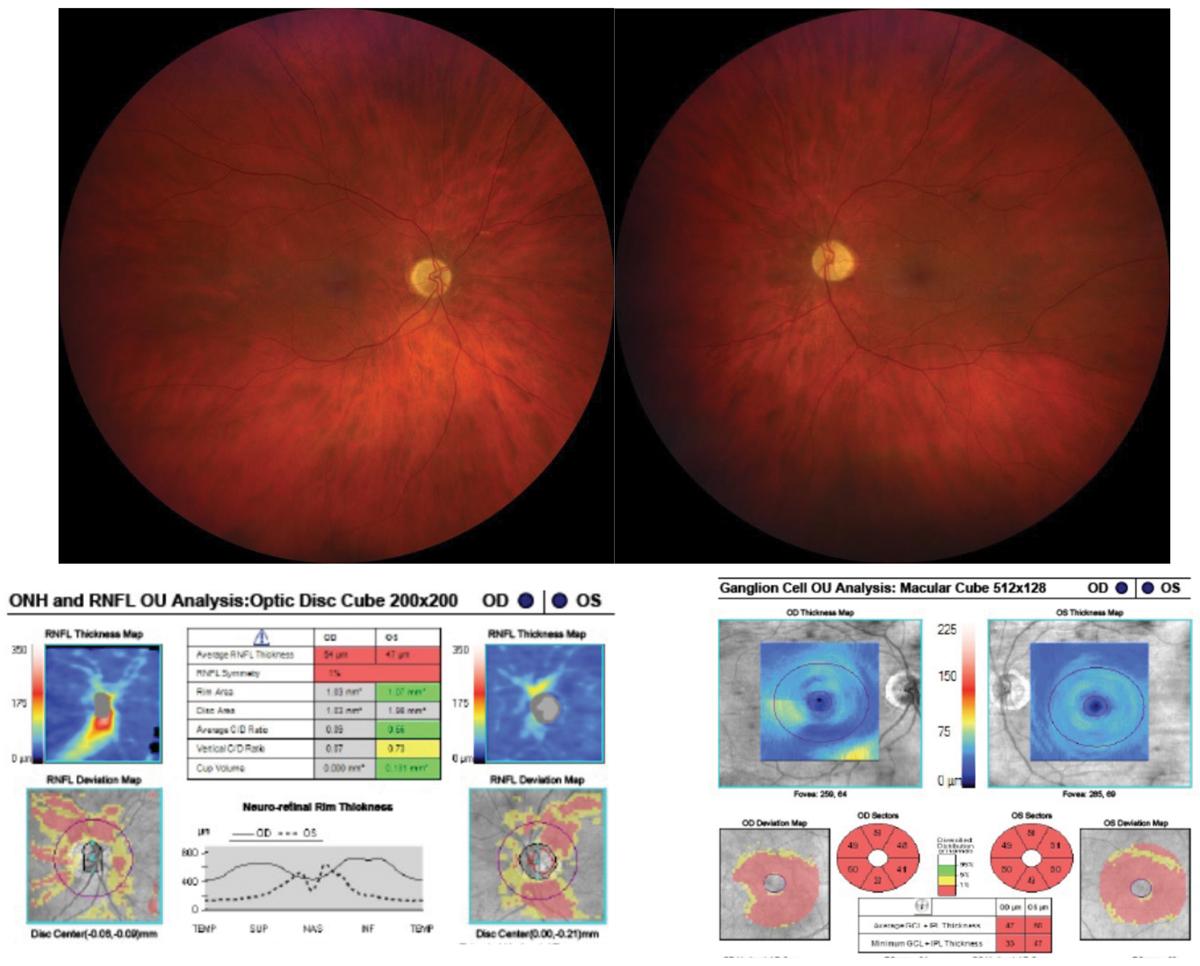
Fig. 4. Bilateral optic disc edema due to CON secondary to sphenoid wing meningioma. OCT reveals intact retinal nerve fiber and ganglion cell layers. Click image to enlarge. |
Inflammatory Optic Neuropathy
Optic neuritis (ON) is a generic term used to describe an acute inflammatory syndrome of the central nervous system that affects the optic nerve.11,25-27 Demyelinating ON is caused by an inflammatory attack that results in axonal injury and retinal ganglion cell apoptosis. It is the most common type of inflammatory disorder of the optic nerve. Typical demyelinating ON carries an increased future risk of multiple sclerosis (MS). This condition also frequently causes optic neuritis. The Optic Neuritis Treatment Trial showed that approximately 50% of ON patients had converted to MS after a 15-year follow-up period.25-27
It is important to note that MS is not the only demyelinating condition that can cause ON. Neuromyelitis optica (NMO) is a rare demyelinating autoimmune inflammatory disease that affects the central nervous system, causing ON and myelitis.25-27 The frequency of NMO is low when compared with MS, with 1/100 cases in North America. Like MS, NMO initial ophthalmic presentations are common, but NMO has more severe bilateral visual disability and optic nerve damage with a guarded visual prognosis. OCT, brain and spinal cord MRI and the titer of anti-aquaporin 4 antibodies are the tests used to distinguish MS from NMO.21-27
Even less common is a recently defined inflammatory demyelinating disease of the central nervous system called myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). This autoimmune disorder is associated with antibodies directed against the myelin of the brain, spinal cord and optic nerves.28 MOGAD may cause ON, transverse myelitis and, in children, acute disseminated encephalomyelitis. Diagnosis is confirmed when the protein’s antibody is found in serum and/or CSF and the presence of MRI phenotype is consistent with MOGAD.28
In acute demyelinating ON, patients present with vision loss in one eye that rapidly worsens for up to two weeks. Periocular and retrobulbar pain occurs in 90% of patients and reportedly worsens with eye movement. Most patients have reduced contrast sensitivity, dyschromatopsia and a visual field defect.25-27 At times, no other neurologic symptoms may exist on presentation. Taking a thorough medical history may uncover past transient, spontaneous-resolving episodes of neurologic dysfunction. For example, a patient may report limb weakness that lasts for days or weeks then resolves or episodic unexplained vertigo or balance loss.25-27
 |
|
Fig. 5. Bilateral optic disc edema associated with IIH. OCT confirms the presence of optic disc edema and RNFL thinning. Click image to enlarge. |
Signs include a variable decrease in VA, contrast sensitivity and color vision. Central scotomas and diffuse visual field loss are common. In unilateral cases, the symptomatic eye has an RAPD. Assessment of the optic nerve, in the minority of cases, will reveal mild to moderate hyperemic disc swelling. Depending on the underlying etiology, peripheral retinal assessment may show signs of intraocular inflammation that include peripheral sheathing of retinal veins and “snow banking,” the accumulation of vitreous exudates over the pars plana.11,25-27
The standard treatment of typical ON is based on results from the Optic Neuritis Treatment Trial, which found that one gram of intravenous (IV) methylprednisolone daily for three days followed by oral prednisolone (1mg/kg/day) for 11 days often sped up visual recovery.25-27 Although the recovery of acute visual loss was sped up, final recovery was the same whether or not steroids were used. A benefit of this regimen of care was that the onset of MS was delayed for up to two years; however, at two years, equal numbers of treated and untreated cases developed MS.25-27
The Controlled High Risk Avonex Multiple Sclerosis Study found that cases of first-episode typical ON with two or more white matter lesions on a brain MRI, when treated with weekly intramuscular beta-interferon injections, had a decreased risk of developing symptoms of MS by three years. Study patients also received the protocol of IV and oral steroids.25-27
Monoclonal antibody agents have been approved by the FDA for adults with demyelinating disease. Those to treat MS include alemtuzumab, natalizumab, ocrelizumab, ofatumumab and rituximab. Approved to treat NMO are eculizumab, inebilizumab and satralizumab, all of which display the ability to significantly reduce the risk of NMO relapse.26,27
Vascular Optic Neuropathy
One such condition—ischemic optic neuropathy (ION)—is the result of a transient or permanent interruption of blood supply to any portion of the optic nerve.11,29-31 Anterior ION (AION) involves ischemia of the optic nerve head while posterior ION (PION) involves ischemia of the posterior optic nerve. Further, ION is classified as arteritic or non-arteritic.
The postulated pathophysiologic mechanism of non-arteritic AION (NAION) involves the prelaminar portion of the optic nerve head.29,31 The short posterior ciliary arteries (PCAs) are thought to be a component of a compartment syndrome of the prelaminar nerve. A structurally predisposed, crowded optic nerve head with a small cup-to-disc ratio, referred to as “a disc at risk,” may lead to the death of ganglion cells. Vascular insufficiency is thought to play a role in the pathophysiology of NAION, as vascular diseases such as hypertension, diabetes and obstructive sleep apnea impair autoregulation of the optic nerve head blood flow.29,31
 |
|
Fig. 6. Blurred disc margins with associated hemorrhage secondary to arteritic AION. Click image to enlarge. |
NAION is the most common cause of acute optic nerve disease in patients over 50.29-31 The annual incidence of NAION is 10.3 per 100,00 individuals. The median age of onset is 72, and the condition disproportionately affects those of Caucasian descent.31,32 Clinical features include the sudden onset of rapidly-progressive, painless loss of vision in one eye. Vision loss may be diffusely blurred or in a vertical hemifield distribution, commonly inferiorly. VA loss may vary, with approximately half of patients seeing better than 20/64 and one in three seeing less than 20/200.29-32 Color vision impairment is proportionate to the level of acuity loss. Optic disc swelling may be diffuse or sectoral and accompanied by peripapillary flame hemorrhages. An RAPD is expected in optic neuropathy that is unilateral.31,32
Non-arteritic PION is a rare entity that presents with optic nerve signs and symptoms in the absence of disc edema. A diagnosis of exclusion, non-arteritic PION can only be diagnosed in the context of a normal MRI of the orbits, ruling out a compressive cause, and when giant cell arteritis (GCA) is excluded in patients over 50.11,29-31
Treatment of NAION, in theory, would reduce the optic nerve compartment syndrome via surgical decompression or by reducing disc edema. The Ischemic Optic Neuropathy Decompression Trial ceased recruitment when preliminary findings suggested no benefit and potential harm related to surgery.33 Further investigations included the use of intravitreal bevacizumab, oral prednisolone and aspirin, all of which proved to be of no benefit in the recovery of VA.29,31,33 An advised strategy to reduce the lifetime risk of further episodes of NAION includes the treatment of vascular risk factors, including hypertension, diabetes and obstructive sleep apnea, as well as smoking cessation.29,31,33
Arteritic AION is almost always caused by GCA, a large-vessel vasculitis affecting people over the age of 50.11,29-31 Up to 20% of patients with giant cell arteritis have AION, a form of end-organ ischemia. Thrombotic occlusion of the short PCAs because of large-vessel vasculitis causes the optic nerve head infarction seen in AION (Figures 6 and 7). The diagnosis of GCA may be confirmed by erythrocyte sedimentation rate, C-reactive protein and temporal artery biopsy.29-31
Signs are commonly preceded by transient monocular vision loss resulting from optic nerve or choroidal ischemia. Transient monocular vision loss is a medical emergency warranting medical workup to rule out, among others, GCA and cerebral vascular accident.29-31 Transient diplopia due to extraocular muscle or cranial nerve ischemia may be reported. The large-vessel vasculitis associated with GCA may cause symptoms of jaw claudication, scalp tenderness, headache, fever, malaise and weight loss.29-31
Modified Dandy Criteria to Identify IIH • Signs and symptoms of increased ICP • Absence of localized findings on neurologic examination • Absence of deformity, displacement or obstruction of the ventricular system and otherwise normal neurodiagnostic studies except for evidence of increased CSF pressure >200mm H2O. Abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled-out CSF spaces and smooth-walled, non-flow-related venous sinus stenosis or collapse should lead to another diagnosis. • Awake and alert • No other known cause of increased ICP, opening CSF pressure of 200mm H2O to 250mm H2O and at least one of the following: - Pulse synchronous tinnitus - Cranial nerve VI palsy - Frisen grade 2 papilledema - Echography negative for drusen and no other disc anomalies mimicking disc drusen - Magnetic resonance venography with lateral sinus collapse or stenosis - Partially empty sella on coronal or sagital views and optic nerve sheaths with filled-out CSF spaces next to the globe on T2-weighted axial scans |
Clinical findings associated with arteritic AION include severe visual acuity loss of worse than 20/200 in over 60% of patients with a relative afferent pupillary defect. Posterior segment assessment may reveal signs of ocular ischemia in the form of pallid or chalky white disc edema and retinal cotton wool spots.11,29-33
Acute management of anterior ischemic optic neuropathy includes high-dose glucocorticoids, including prednisolone or IV methylprednisolone.27-30 Specific to GCA, IV steroids are indicated based on improved visual outcomes, potential evolution of systemic complications and prevention of second-eye involvement.27,29 Tocilizumab is a monoclonal antibody agent that is used in conjunction with corticosteroids, providing a higher probability of sustained GCA remission and vision preservation.34
Infectious Optic Neuropathy
Numerous infectious agents have been identified as known causes of optic neuropathy. Bacteria, spirochetes, fungi and viruses may directly and indirectly cause inflammatory, degenerative or vascular compromise of the optic nerve.11,35,36 Infectious optic neuropathy may present as an anterior optic neuritis (papillitis), retrobulbar optic neuritis (optic disc is normal in appearance), neuroretinitis (optic disc edema with macular star of exudate), anterior ischemic optic neuropathy or a perineuritis (infection affects the sheath of the optic nerve causing optic disc swelling).35,36
The most common bacteria include Bartonella henselae (cat scratch disease), Mycobacterium tuberculosis (tuberculosis), Treponema pallidum (syphilis) and Borrelia burgdorferi (Lyme disease).35.36 Viral causes include herpes simplex virus types 1 and 2, varicella zoster virus (that includes both varicella and herpes zoster), cytomegalovirus, Epstein-Barr virus (mononucleosis) and human immunodeficiency virus.35,36 Other infections due to parasitic vectors, toxoplasmosis and toxocariasis, and fungal vectors, histoplasmosis, may also cause optic neuropathy.35,36
The prognosis for recovery of visual function is largely dependent on timely assessment, ordering proper diagnostic tests and providing targeted medical treatments. In cases of infectious optic neuropathy, OCT test results may reveal damage incurred at the retinal nerve fiber and ganglion cell layers, limiting potential recovery of visual function.35,36
Toxic, Nutritional Optic Neuropathy
These disorders occur from persistent exposure to a toxic substance or a sustained nutrient deficiency.11,37 Toxic neuropathy occurs due to the use of toxic medications, as well as ingestion or inhalation of toxic substances. Medication toxicity is both dose-dependent and duration-dependent. The antimycobacterial drug, ethambutol, is the most encountered cause of toxic optic neuropathy.37 Methanol optic neuropathy has an acute clinical picture when ethanol is injected. More commonly, neuropathy occurs when ethanol in home-distilled alcoholic beverages is inadvertently ingested. The antiarrhythmic drug, amiodarone, has been associated with optic neuropathy mimicking NAION.37-39
Nutritional optic neuropathy has a well-established association with vitamin B12, folic acid and copper deficiencies.37-39 Pernicious anemia, nitrous oxide toxicity and gastric maladies like atrophic gastritis, history of gastric surgery, gastric malabsorption and treatment of gastroesophageal reflux are all potential causes of B12 deficiencies. Folate deficiency is the result of decreased uptake, increased demand, malabsorption or suppression due to a folate antagonist, such as methotrexate. Copper deficiency is most commonly caused by gastric surgery and the associated malabsorption syndrome.37-39
Tobacco and alcohol use have been considered to have an association with toxic optic neuropathy.37-39 Alcohol is not considered to be a direct cause of toxic optic neuropathy. Alcoholism and its association with a higher incidence of nutritional deficiencies and gastric malabsorption can result in optic neuropathy. Toxic optic neuropathy attributed to smoking is a diagnosis of exclusion.37,39
Early in the development of toxic and nutritional optic neuropathy, the optic nerves may have a normal appearance or may be slightly hyperemic.38,39 If the exposure or deficiency persists, bilateral temporal optic disc pallor develops from injury of the ganglion cell axons in the papillomacular bundle. Visual field testing results in bilateral central and cecocentral defects. Progressive, bilateral vision loss, decreased color vision and normal pupil assessment (due to symmetry of optic nerve damage) may be present.38,39
 |
|
Fig. 7. Bilateral optic disc pallor secondary to toxic optic neuropathy due to rheumatologic medication. Discs are pale, and OCT confirms RNFL and GCC defects. Click image to enlarge. |
Traumatic Optic Neuropathy
This form of optic neuropathy is caused by injury to the optic nerves. The degree of vision loss depends on the portion of the optic nerve affected by the injury.11,40-42 The classification of traumatic optic neuropathy can be described as either primary or secondary. Primary lesions may be further described as direct (penetrating) or indirect (non-penetrating, blunt trauma). Direct injuries are less common because of the protection provided by the orbit. When they do occur, direct injuries result in immediate and often irreversible damage to the affected nerve.40-42
The mechanism by which indirect injury may occur includes transmission of concussive forces directly to the nerve, as in cases involving the portion of the nerve within the optic canal.40-42 These forces may also induce the transmission of energy away from the point of impact, generating rotational or translational movement of the globe or brain.40-42
A secondary traumatic optic neuropathy is due to damage to the optic nerve that occurs after the traumatic event. In these cases, visual loss is delayed.32-34 There are several proposed mechanisms of how secondary injury may occur, including vasospasm, edema, hemorrhage and compression of vessels causing circulatory insufficiency and resulting in necrosis of the nerve.40-42
Takeaways
Optic nerve disorders are a relatively common clinical finding and are the result of many congenital, hereditary and acquired diagnoses. In cases of acquired optic neuropathy, it is important to distinguish between glaucomatous and non-glaucomatous etiologies. Awareness of a chronic or acute pattern of presentation, along with a detailed history, constellation of clinical findings and results of structural and functional diagnostic studies, allow for proper diagnosis and treatment.
Properly identifying optic neuropathies can not only save vision but also lives.
Dr. Myers is a senior staff optometrist at the Coatesville Veterans Affairs Medical Center in Coatesville, PA. He has served as a guest lecturer and adjunct clinical faculty at the Pennsylvania College of Optometry at Salus University in Philadelphia, PA.
Dr. Gurwood is a professor at the Pennsylvania College of Optometry at Salus University. He is an attending staff member of the Department of Ophthalmology at the Albert Einstein Medical Center in Philadelphia. Drs. Myers and Gurwood have no financial interests to disclose.
1. Edward DP, Kaufman LM. Anatomy, development, and physiology of the visual system. Pediatr Clin North Am. 2003;50(1):1-23. 2. Freddi TAL, Ottaiano AC. The optic nerve: Anatomy and pathology. Semin Ultrasound CT MR. 2022;43(5):378-88. 3. Nicholson B, Ahmad B, Sears JE. Congenital nerve malformation. Inter Ophthalmol Clin. 2011;51(1):49-76. 4. Heidary G. Congenital optic nerve anomalies and hereditary optic neuropathies. J Pediatr Genet. 2014;3(4):271-80. 5. Lei K, Qu Y, Tang Y, et al. Discriminating between compressive optic neuropathy with glaucoma-like cupping and glaucomatous optic neuropathy using OCT and OCTA. Transl Vis Sci Technol. 2023;12(3):1-11. 6. Dallorto L, Lavia C, Jeannerot AL, et al. Retinal microvasculature pituitary adenoma patients: is optical coherence tomography angiography useful? Acta Ophthalmol. 2020;98(5):e585-92. 7. Laowanapiban P, Sathianvichitr K, Chirapapaisan N. Structural and functional differentiation between compressive and glaucomatous optic neuropathy. Sci Rep. 2022;12(1):6795. 8. Waisberg E, Micieli JA. Neuro-ophthalmological optic nerve cupping: an overview. Eye Brain. 2021;13:255-68. 9. Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 2020;127(4): S45-S69. 10. Coleman-Belin J, Harris A, Chen B, et al. Aging effects on optic nerve neurodegeneration. Int J Mol Sci. 2023;24:2573. 11. Dworak DP, Nichols J. A review of optic neuropathies. Dis Mon.2014;60(6):276-81. 12. Smith MM, Strottmann JM. Imaging of the optic nerve and visual pathways. Semin Ultrasound CT MR. 2001;22(6):473-87. 13. Biousse V, Danesh-Meyer HV, Saindane AM, et al. Imaging of the optic nerve: technological advances and future prospects. Lancet Neurol. 2022;21(12):1135-50. 14. Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr Opin Neurol. 2019;32(1):115-23. 15. Liu A, Craver EC, Bhatti MT, et al. Population based incidence and outcomes of compressive optic neuropthy. Am J Ophthalmol. 2022;236(4):130-5. 16. Henaux PL, Bretonnier M, Le Reste PJ, et al. Modern management of meningiomas compressing the optic nerve: A systematic review. World Neurosug. 2018;118:e677-86. 17. Algoet M, Van Dyck-Lippens PJ, Casselman J, et al. Intracanal optic nerve cavernous hemangioma: A case report and review of the literature. World Neurosurg. 2019;126:428-33. 18. Reier L, Fowler JB, Arshad M, et al. Optic disc edema and elevated intracranial pressure (ICP): A comprehensive review of papilledema. Cureus. 2022;14(5):e24915. 19. Rigi M, Almarzouqi SJ, Morgan ML, et al. Papilledema: epidemiology, etiology, and clinical management. Eye Brain. 2015;7:47-57. 20. Crum OM, Kilgore KP, Sharma R, et al. Etiology of papilledema in patients in the eye clinic setting. JAMA Netw Open. 2020;3:e206625. 21. Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: profile at baseline. JAMA Neurol. 2014;71:693-701 22. Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088-1100. 23. Daggubati LC, Liu KC. Intracranial venous sinus stenting: a review of idiopathic intracranial hypertension and expanding indications. Cereus. 2019;11:e4008. 24. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-65. 25. Hickman SJ, Petzold A. Update on Optic Neuritis: an international view. Neuroophthalmology. 2021;46(1):1-18. 26. de Seze J. Inflammatory optic neuritis: from multiple sclerosis to neuromyelitis optica. Neuroophthalmology. 2013;37(4):141-5. 27. Boudreault K, Durand ML, Rizzo JF. Investigation-directed approach to inflammatory optic neuropathies. Semin Ophthalmol. 2016;31(1-2):117-30. 28. Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A review of clinical and MRI features, diagnosis, and managent. Frontiers in Neurology 2022;13(6):1-21. 29. Patil AD, Biousse V, Newman NJ. Ischemic optic neuropathies: current concepts. Ann Indian Acad Neurol. 2022;25(Suppl 2):S54-8. 30. Dotan G, Korczyn AD. Nonarteritic ischemic optic neuropathy and other vascular diseases. Neuroepidemiology. 2013;40:225-6. 31. Augstburger E, Heron E, Abanou A, et al. Acute ischemic optic nerve disease: pathophysiology, clinical features and management. J Fr Ophthalmol. 2020;43(2):e41-e54. 32. Banc A, Kupersmith M, Newman NJ, et al. Race distribution in non-arteritic anterior ischemic optic neuropathy. Am J Ophthalmol. doi:10.1016/j.ajo.2023.03.013. 33. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625-32. 34. Bouffard MA, Prasad S, Unizony S, et al. Does Tocilizumab influence ophthalmic outcomes in giant cell arteritis? J Neuro-Ophthalmol 2022;42:173-179. 35. Golnik KC. Infectious optic neuropathy. Semin Ophthalmol. 2002;17(1):11-17. 36. Kahlun R, Abroug N, Ksiaa I, et al. Infectious optic neuropathies: a clinical update. Eye Brain. 2015;7:59-81. 37. Margolin E, Blair K, Shemesh A. Toxic and nutritional optic neuropathy. StatPearls Publishing. 2022; https://www.ncbi.nlm.nih.gov/books/NBK499979/. 38. Grzybowski A, Zulsdorff M, Wilhelm H, et al. Toxic optic neuropathies: an updated review. Acta Opthalmol. 2015;93(5):402-10. 39. Baj J, Forma A, Kobak J, et al. Toxic and nutritional optic neuropathies – an updated mini-review. Int J Environ Res Pub Health. 2022;19(5):3092. 40. Miller NR. Traumatic optic neuropathy. J Neurol Surg B Skull Base. 2021;82(1):107-15. 41. Chen B, Zhang H, Zhai Q, et al. Traumatic optic neuropathy: a review of current studies. Neurosurg Rev. 2022;45(3):1895-1913. 42. Au NPB, Ma CH. Neuroinflammation, microglia and implications for retinal ganglion cell survival and axon regeneration in traumatic optic neuropathy. Front Immunol. 2022;13:860070. |
