 |
Conjunctivitis: Know Your Differentials
The various forms of this condition display similar signs and symptoms, which can make a specific diagnosis challenging.
By Marc Bloomenstein, OD
Release Date: November 15, 2022
Expiration Date: November 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
Recognize the key features of different types of conjunctivitis.
Accurately diagnose the specific cause of conjunctivitis.
Differentiate between allergic, viral and bacterial conjunctivitis.
Educate their patients on the underlying cause of their condition.
Target Audience: This activity is intended for optometrists engaged in conjunctivitis management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Marc Bloomenstein, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #124938 and course ID 81535-TD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Author: Dr. Bloomenstein has no financial interests to disclose. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
Commonly described as “pink eye,” conjunctivitis is a broad clinical term that includes a variety of infectious and noninfectious conditions. Typically it is characterized by inflammation and swelling of the conjunctival tissue, accompanied by engorgement of the blood vessels, ocular discharge and occasional pain.1 More than 80% of all acute cases of conjunctivitis are reported to be diagnosed by non-ophthalmic clinicians, including internists, family medicine physicians, pediatricians and nurse practitioners, which makes it even more important for the ophthalmic community to be the final voice in the diagnosis.2
There are several ways to categorize conjunctivitis: by etiology, chronicity, severity and extent of involvement of the surrounding tissue. The etiology of conjunctivitis may be infectious or noninfectious, affecting people of any age, race or socioeconomic status. A challenge for clinicians is differentiating other causes of “red eye” associated with severe sight- or life-threatening consequences. Since conjunctivitis may be associated with the involvement of the surrounding tissue, such as the eyelid margins and cornea, the diagnosis is not always straightforward.
Further adding to the importance of a timely diagnosis is the association between conjunctivitis and systemic conditions, including immune-related diseases (e.g., Reiter’s, Stevens-Johnson syndrome and keratoconjunctivitis sicca in rheumatoid arthritis), nutritional deprivation (vitamin A deficiency) and congenital metabolic syndromes (Richner-Hanhart syndrome and porphyria).3,4 This article will delve into the different types of conjunctivitis to help optometrists narrow down the differentials and reach the correct diagnosis.
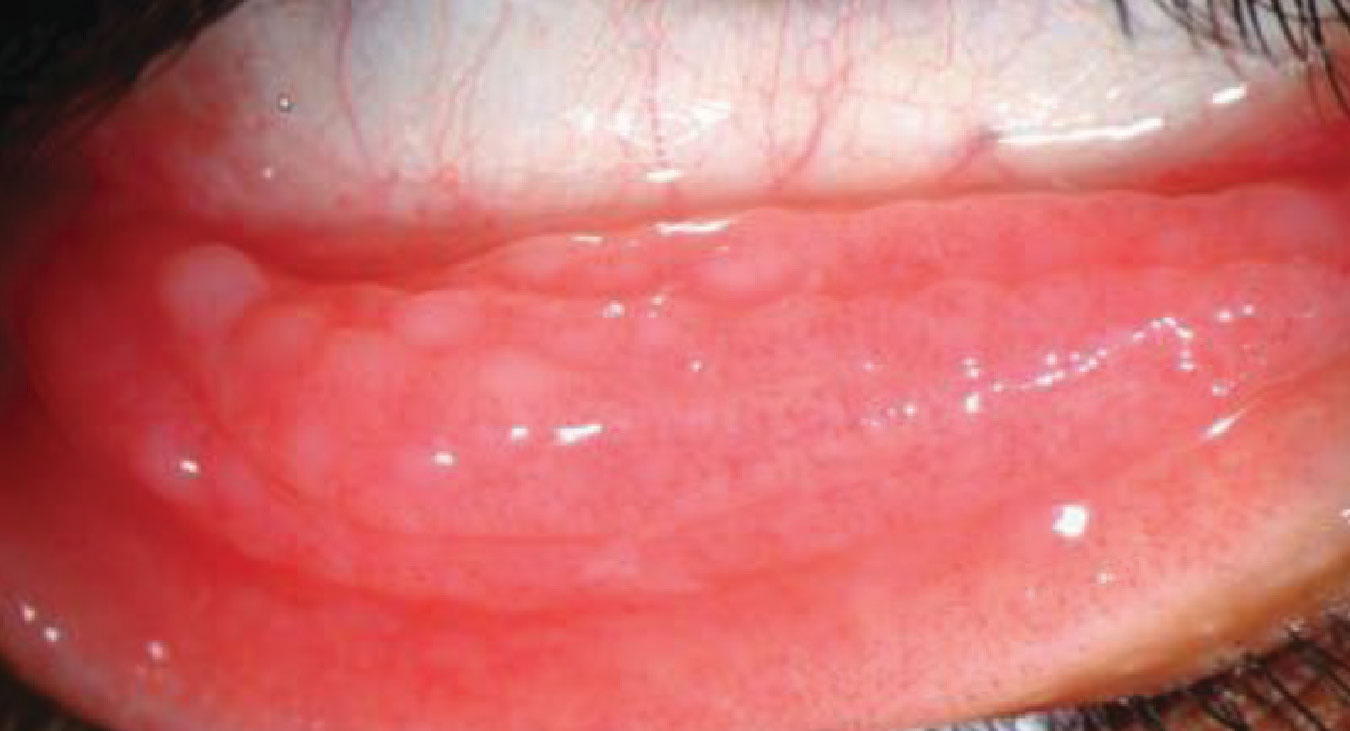 |
| VKC is a more severe form of conjunctivitis and is most likely allergic in origin. Photo: Christine Sindt, OD. Click image to enlarge. |
Diagnosing Conjunctivitis
This condition is characterized, but not limited to, conjunctival hyperemia, ocular discharge and, depending on the etiology, discomfort and itching, with differing signs and symptoms. In a large meta-analysis, anisocoria and mild photophobia were significantly associated with non-conjunctivitis origins.5 The presence of these two signs could help diagnose 59% of cases, including those associated with anterior uveitis and keratitis.
Aside from the more common signs associated with conjunctivitis—discharge, conjunctival injection, mucus, grittiness, edema—a thorough history can guide the clinical diagnosis.6 A focused ocular history should include the following: onset and duration of symptoms, laterality, impairment of vision, presence of itch, contact lens wear, comorbidities such as infection, sinusitis and lymphadenopathy, previous episodes of conjunctivitis, systemic allergies and medication, as well as a history of exposure to chemical agents.
Associated symptom history, such as fever, malaise, fatigue and contact with individuals who have conjunctivitis, helps to define the differential diagnosis. SARS-CoV-2 should be included in any differential following the 2020 outbreak. Physical examination, including checking for palpable lymph nodes, especially in the periauricular and submandibular areas, is of great importance.
Whereas signs of redness and discharge are most commonly a cause of conjunctivitis, they do not differentiate the pathogen of origin.7 In one study, an accuracy rate of only 48% in making the correct diagnosis of adenoviral conjunctivitis was noted.8 Several other studies demonstrated that bacterial pathogens are only isolated in 50% of cases of suspected bacterial conjunctivitis.9 In addition, one study reported that up to 52% of presumed cases of viral conjunctivitis were culture-positive for bacteria.8
There are generalizations that can be used to differentiate conjunctivitis types. It is commonly believed that involvement of one eye followed by the second eye within 24 to 48 hours is indicative of bacterial infection, and that infection of the second eye after 48 hours should raise suspicion for a viral etiology.8 Further, a papillary conjunctival reaction or pseudomembranous conjunctivitis suggests a bacterial origin, whereas a follicular conjunctival reaction is more likely to indicate a viral etiology. However, caution should be exercised when using these signs to make a definitive diagnosis.
It is also loosely believed that an association between lack of itching and bacterial conjunctivitis, as well as recent upper respiratory tract infection and lymphadenopathy, favors a viral conjunctivitis: sinusitis. However, a 2003 meta-analysis failed to find any clinical studies correlating the signs and symptoms of conjunctivitis with its underlying etiology.10 Furthermore, a prospective study found the strongest predictors of bacterial conjunctivitis are bilateral matting of the eyelids, lack of itching and no previous history of conjunctivitis.11 It was noted that the types of discharge (purulent, mucus or watery) or other symptoms were not specific to any particular class of conjunctivitis. In the case of herpes simplex virus, the lack of bilaterality can also be an indicator since the bilateral nature is uncommon.
Acute conjunctivitis of all causes is estimated to occur in six million Americans annually.12 The highest rates are among children who are younger than seven years old, with the highest incidence occurring between birth and age four. Allergic conjunctivitis, affecting 15% to 40% of the population, is the most common type and is seen most often in the spring and summer. Acute bacterial conjunctivitis is the second most common, and its rates are highest from December to April.1
Conjunctival cultures are generally reserved for cases of suspected infectious neonatal conjunctivitis, recurrent conjunctivitis, conjunctivitis recalcitrant to therapy, conjunctivitis presenting with severe purulent discharge and cases suspicious for gonococcal or chlamydial infection.13 Although primary studies from in-office rapid antigen testing for adenoviruses report 89% sensitivity and up to 94% specificity, the results of more recent studies point toward a high specificity but only moderate sensitivity ranging from 40% to 50%.13,14 Accordingly, it may be suggested that negative test results should be confirmed by real-time PCR owing to the test’s suboptimal sensitivity.
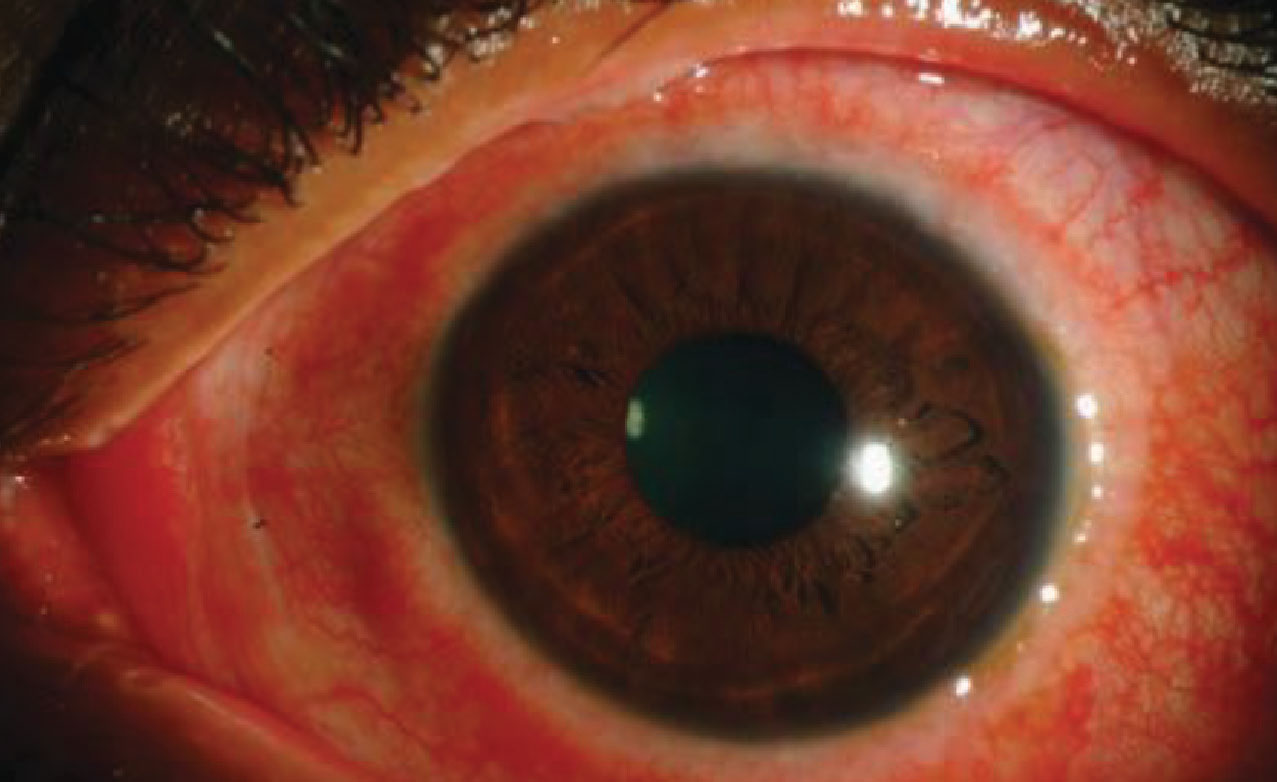 |
| Viral conjunctivitis is often an acute, contagious conjunctival infection related to an infection of the upper respiratory tract or an adenovirus. Symptoms, which are usually limited to one eye at a time, include irritation, photophobia and watery discharge. Photo: Christine Sindt, OD. Click image to enlarge. |
Viral Conjunctivitis
This is the most common cause of infectious conjunctivitis, causing up to 80% of all acute cases, with many misdiagnosed as bacterial conjunctivitis.15 Between 65% and 90% of viral conjunctivitis cases are caused by adenoviruses, and they produce the three most common presentations associated with this conjunctivitis: follicular conjunctivitis, pharyngoconjunctival fever and epidemic keratoconjunctivitis.16,17 Let’s review each in turn.
Follicular conjunctivitis is the mildest form of a viral conjunctival infection. It has an acute onset, initially unilateral with the second eye becoming involved after about a week. It presents with a watery discharge, hyperemia, follicular reaction and a preauricular lymphadenopathy on the affected side. Most cases resolve spontaneously.18
The most common form of adenovirus infection in children is pharyngoconjunctival fever caused by HAdV types 3, 4 and 7.19-21 This condition is usually characterized by the presence of fever, pharyngitis, periauricular lymphadenopathy and acute follicular conjunctivitis. Ocular findings include edema, hyperemia and petechial hemorrhages of the conjunctiva.20 This condition is self-limited, often resolving spontaneously in two to three weeks without any treatment. Patients should be educated as to the contagious nature of these viruses, use proper hygiene and avoid direct contact during the contagious period.
The most severe ocular manifestation of adenoviral infection is epidemic keratoconjunctivitis (EKC), affecting both the conjunctiva and cornea, with the potential to leave long-lasting, permanent ocular surface changes and visual disturbances. Ocular manifestations of EKC include conjunctival discharge, follicular conjunctivitis, corneal subepithelial infiltrates (SEIs), corneal scarring, conjunctival membranes and pseudomembranes and symblepharon formation. Pseudomembranes, which are sheets of fibrin-rich exudates without blood or lymphatic vessels, may be encountered in the tarsal conjunctiva of the EKC patient.22 Depending on the intensity of inflammation, true conjunctival membranes may also form in EKC. True membranes, once formed, can lead to the development of subepithelial fibrosis and symblepharon.23
Timely diagnosis of these adenoviruses is critical, as the replication of the virus in the corneal epithelium may cause superficial punctate keratopathy, followed by focal areas of epithelial opacities.24 Focal SEI in the anterior stroma of the cornea appears approximately seven to 10 days following the initial involvement of the eye with EKC.25 These opacities may persist for years, and they can be associated with visual disturbance, photophobia and astigmatism. The incidence of SEI formation in EKC has been reported to vary from 49% to 80%.26 It is believed that an immunologic reaction to the replicating adenoviruses in the anterior stromal keratocytes leads to the formation of SEIs. In fact, the recurrence of SEIs following discontinuation of steroids is a strong indicator of this theory.27
Adenovirus conjunctivitis is very contagious, with reports showing it may be transmitted up to 50% of the time.28 The virus is most commonly spread from the hands. As many as 46% of individuals with viral conjunctivitis had positive viral culture grown from their hands, according to one study. The virus may also spread through office equipment, swimming pools or sharing personal items.29 The incubation period for the adenovirus is approximately five to 12 days, while the infected individual can transmit the disease for up to 14 days from the time they are infected.28 With such high transmissions rates, the use of gloves and hand washing is imperative within the office.
There is no single effective treatment modality for viral conjunctivitis; however, palliative treatment is recommended.30 Off-label topical ganciclovir has been used against EKC, showing potential against specific adenovirus serotypes in vitro.9 One study compared the effects of ganciclovir 0.15% ophthalmic ointment with preservative-free artificial tears for 18 patients with adenovirus keratoconjunctivitis.31 The ganciclovir group demonstrated resolution of the conjunctivitis in 7.7 days as opposed to 18.5 days for the artificial tear group. Additionally, topical antibiotics do not play a role in treating viral conjunctivitis. Most potentially obscure the clinical picture by inducing ocular surface toxicity, increasing bacterial resistance and spreading the disease to the contralateral eye by cross-contamination through the infected bottles.8,32
A monotherapy against viral conjunctivitis with povidone-iodine 2%, a broad-spectrum antimicrobial with high microbial kill rates, has been investigated. Researchers found that the topical administration of povidone-iodine 2% four times a day for one week led to complete resolution of the disease in three-quarters of the study eyes.33 Varying concentrations of povidone-iodine/dexamethasone suspension have also been used, and the results suggest that the combination therapies reduce patient symptoms and eradicate the virus effectively.34 Additionally, the use of cyclosporin or cyclosporine A eye drops has been suggested to help treat corneal infiltrates.35
Although not as common as adenoviruses, herpes simplex is estimated to cause 1.3% to 4.8% of all cases of viral conjunctivitis.36 The zoster virus has also been shown to induce conjunctival involvement, rarely with corneal involvement.
Acute hemorrhagic conjunctivitis is another extremely contagious virus. It manifests through foreign body sensation, profuse tearing, eyelid edema, dilatation of conjunctival vessels, chemosis and the hallmark sign of subconjunctival hemorrhage.37 Be weary of the monocular viral infection. Since—as previously discussed—viral infections tend to be bilateral, a unilateral presentation should elicit further testing in-clinic.
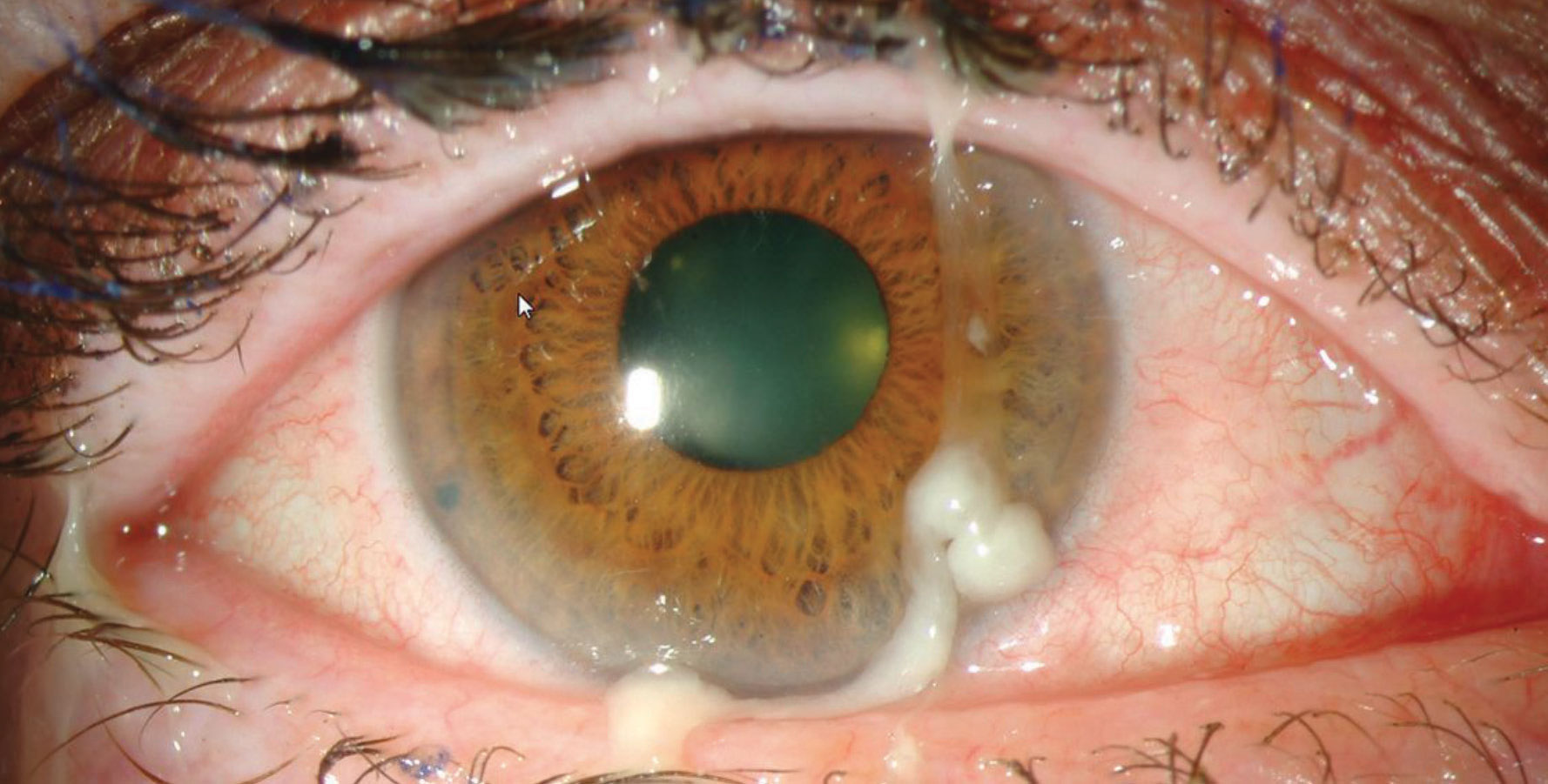 |
| Bacterial conjunctivitis is often a byproduct of the natural flora of the individual and is most commonly caused by Staphylococcus aureus, Streptococcus pneumonia and Haemophilus. Photo: Christine Sindt, OD. Click image to enlarge. |
Bacterial Conjunctivitis
Among adults, this condition is less common than viral conjunctivitis. However, in children it is encountered more frequently, specifically in the form of Haemophilus influenzae.38 Bacterial conjunctivitis can result from either direct contact with infected individuals or from abnormal proliferation of the native conjunctival flora.
Acute bacterial conjunctivitis is most often caused by Staphylococcus species, Haemophilus influenzae, Streptococcus species, Moraxella catarrhalis and gram-negative intestinal bacteria.39 In more than 60% of cases, spontaneous resolution occurs within one to two weeks, and serious complications are extremely rare.40,41 However, the presence of a large population of bacteria on the conjunctiva exposes the patient to a higher risk of keratitis, particularly in conditions associated with corneal epithelial defects.39
Topical antibiotics have long been the gold standard in treatment for bacterial conjunctivitis. Although this approach reduces the duration of the disease, no difference in the outcome has been reported between the treatment and placebo groups. In a meta-analysis consisting of 3,673 patients from 11 randomized clinical trials, topical antibiotic treatment increased the rate of clinical improvement by only 10% compared with placebo.42 Furthermore, there is growing resistance to antibiotics and methicillin-resistant S. aureus and total resistance to all β-lactam antimicrobials.43 Suspected cases of MRSA/MRSE need to be treated with fortified vancomycin eye drops or ointments, which are obtainable through a specialty pharmacy that can compound these medications.44
Chlamydial Conjunctivitis
A variety of ocular surface infections can be caused by Chlamydia trachomatis including trachoma, neonatal conjunctivitis and inclusion conjunctivitis. Inclusion conjunctivitis is reported to cause 1.8% to 5.6% of all cases of acute conjunctivitis, where the majority of cases are unilateral and have concurrent genital infection.45,46 Patients often present with mild mucopurulent discharge and follicular conjunctivitis persisting for weeks to months.37 Up to 54% of men and 74% of women are reported to have simultaneous genital infection.47 Treatment with systemic antibiotics such as oral azithromycin and doxycycline is efficacious, while the addition of topical antibiotics is not beneficial.
Trachoma is the leading cause of infectious blindness in the world, affecting 40 million individuals worldwide. This infection is prevalent in areas with poor hygiene. Although mucopurulent discharge is the initial presenting sign, in the later stages, scarring of the eyelids, conjunctiva and cornea may lead to loss of vision. Treatment consists of topical antibiotic ointments, such as tetracycline and erythromycin, in coordination with a systemic antibiotic.48
Gonococcal Conjunctivitis
Typically viewed as a condition affecting neonates, gonococcal conjunctivitis affects other age groups as well. Neisseria gonorrhoeae is a common cause of hyperacute conjunctivitis in neonates and sexually active adults.49 Ocular infection with N. gonorrhoeae is associated with a high prevalence of corneal perforation.39 Gonococcal conjunctivitis should be considered the causative agent in neonates who present with conjunctivitis in days two to five after delivery.50 In both neonatal and non-neonatal populations, conjunctival injection and chemosis, along with copious mucopurulent discharge and a tender globe with periauricular lymphadenopathy, may also be associated with this type of conjunctivitis.50 The suggested treatment for neonates includes systemic management to eradicate the infection.
Allergic Conjunctivitis
Allergy diagnoses have dramatically increased in the last decades secondary to advances in genetics, increased air pollution, foliage, pets and early childhood exposure.51 A study classified ocular allergic conditions into three main categories: IgE-mediated reactions, including seasonal allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC), combined IgE and non-IgE-mediated reactions, including vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), and non-IgE-mediated reactions, including giant papillary conjunctivitis (GPC) and contact dermatoconjunctivitis.
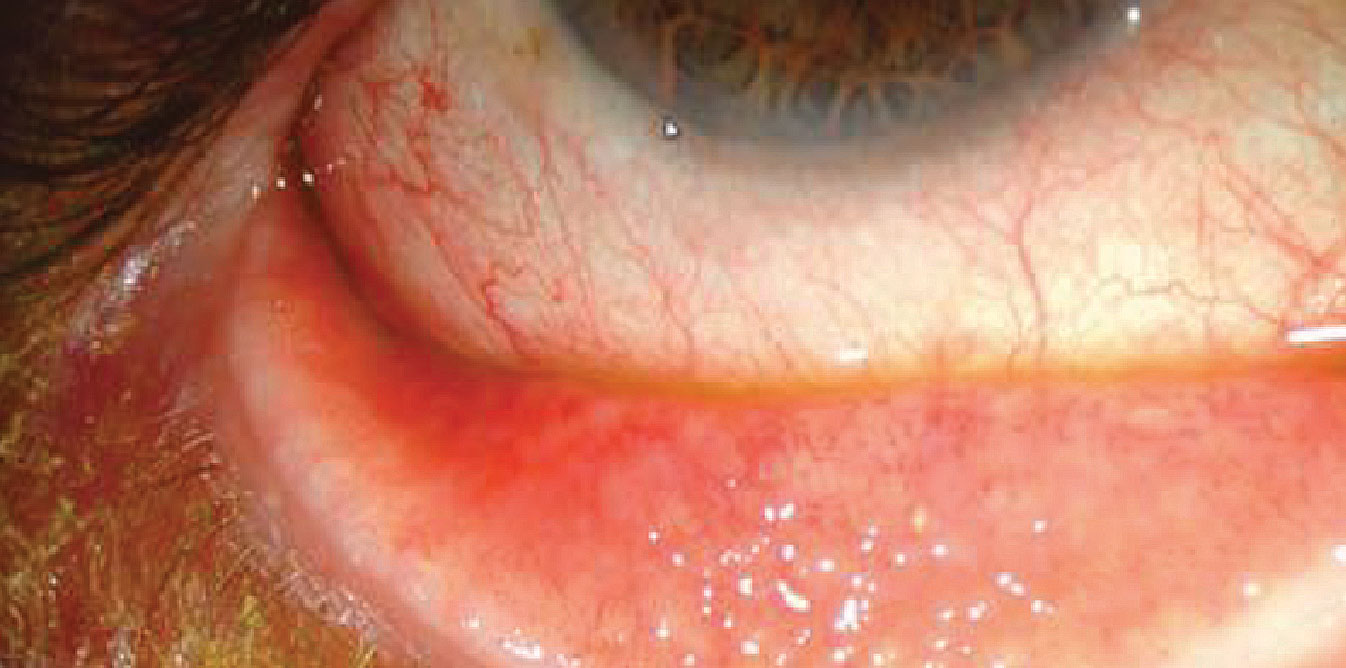 |
| Adult inclusion conjunctivitis is caused by Chlamydia trachomatis and has an incubation period of two to 19 days. Most patients have a unilateral mucopurulent discharge, as well as a follicular and hyperemic tarsal conjunctiva response. Photo: Paul M. Karpecki, OD, and Diana L. Shechtman, OD. Click image to enlarge. |
SAC and PAC
These forms are considered the most prevalent allergic ocular conditions, affecting 15% to 20% of the population.52 The pathogenesis is predominantly an IgE-mediated hypersensitivity reaction, and allergen-specific IgE antibodies are found in almost all cases of SAC and PAC.52 Activation of mast cells contributes to increased levels of histamine, prostaglandins and leukotrienes in the tear film. This early phase lasts 20 to 30 minutes clinically.9 The chemokine release initiates recruitment of inflammatory cells into the conjunctival mucosa, which leads to the late-phase allergic reaction characterized by infiltration of inflammatory cells a few hours after the initial mast cell activation.53
The ocular manifestations of SAC occur predominantly during the spring and summer months when pollens from the trees and plants are released into the air. PAC, on the other hand, can occur throughout the year with exposure to more common allergens such as animal hair, mites and feathers.54 Clinical signs and symptoms are similar in SAC and PAC and include eye itching, burning and tearing. A distinguishing feature is the rare involvement of the cornea.10
VKC
This condition is known as the disease of young males living in warmer climates.55 Although VKC is frequently diagnosed in children, adults can also be affected as well.56 A mixture of IgE and non-IgE reactions in response to nonspecific stimuli, such as wind, dust and sunlight, is often elucidated in this condition. A strong link between VKC and other autoimmune disorders, including atopy, has been suggested.
Conjunctival injection, profuse tearing, severe itching and photophobia are the main clinical signs and symptoms that are associated with VKC. There are three clinical forms of VKC: limbal (limbal papillary reaction and gelatinous thickening of the limbus and Horner-Trantas dots at the superior limbus), palpebral (giant cobblestone papillae) and mixed palpebral and limbal involvement.54
The corneal pathology that is seen in VKC is attributed to the mechanical trauma from the tarsal conjunctival papillae and the inflammatory sequelae of cytokines. In up to 6% of patients, corneal ulcers and plaques develop, leading to exacerbation of clinical symptoms and decreased vision.57 Keratoconus is also highly associated with VKC, affecting nearly 15% of patients with this condition.58
AKC
Characterized by chronic allergic disease of the eyelid, cornea and conjunctiva, AKC is considered the ocular component of atopic dermatitis (AD). Roughly 95% of patients with AKC have concomitant AD.9,12 However, less than 50% with AD have involvement of the ocular tissue.59 Conjunctival cytokines, as well as inflammatory cells, infiltrate the conjunctival tissues in AKC, causing constant remodeling of the ocular surface connective tissue, which leads to mucus metaplasia, scar formation and corneal neovascularization.60
Clinical manifestation of AKC includes epiphora, itching, redness and decreased vision. Presentation is often bilateral; however, unilateral disease has been reported.61 The eyelid skin may be edematous with a sandpaper-like texture. Conjunctival injection and chemosis range from mild to severe, and conjunctival scarring is common.12 Trantas dots and giant papillae may or may not be present. In contrast to VKC, AKC is associated with conjunctival fibrosis and corneal vascularization and opacities. Furthermore “atopic cataracts” are seen at a relatively young age. Shield-like cataracts, as well as nuclear, cortical and even posterior subcapsular cataracts, may also occur. Interestingly, nearly 50% of AKC patients test negative for common allergens.9
GPC
Similar to VKC, this condition is characterized by papillary hypertrophy of the superior tarsal conjunctiva.62 Although GPC is primarily considered a complication of contact lens usage, this condition has also been reported in association with corneal foreign bodies, filtering blebs, ocular prostheses, exposed sutures, limbal dermoids and tissue adhesives.63 The classic signs of GPC consist of excessive mucus secretion associated with decreased contact lens tolerance. Mechanical injuries due to contact lens wear and inflammatory reactions secondary to surface proteins of the lens can contribute to chronic inflammatory damage of the ocular surface.64
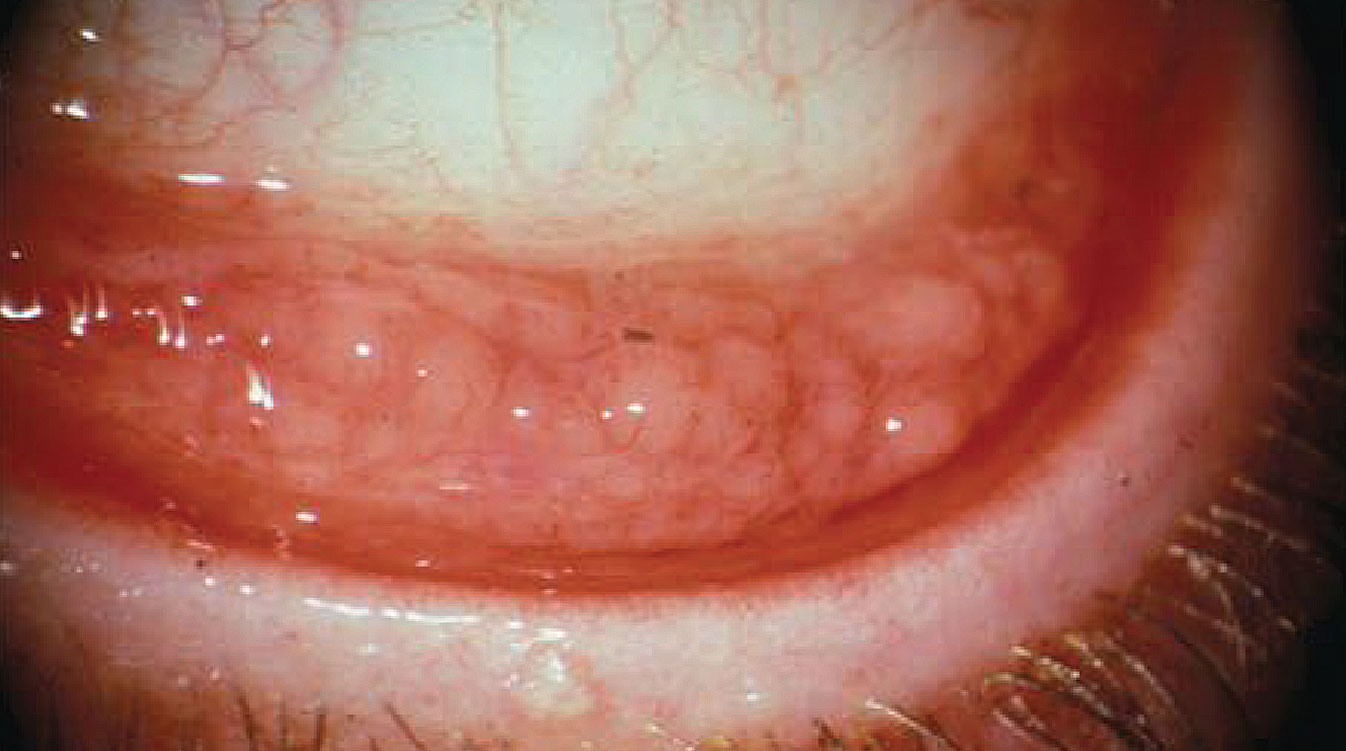 |
| Allergic conjunctivitis is due to a type one hypersensitivity reaction to a specific antigen. Photo: Christine Sindt, OD. Click image to enlarge. |
Allergy Treatment
Avoidance of allergens is the mainstay of treatment for many forms of allergies, including allergic conjunctivitis. Chilled artificial tears provide a barrier function, diluting various allergens and inflammatory mediators. Treatment options for allergic conjunctivitis include lubricating eye drops, antihistamines and mast cell stabilizers.65
Many studies have demonstrated the superiority of topical antihistamines and mast cell stabilizers compared with placebo in alleviating the symptoms of allergic conjunctivitis.66 There are several eyedrop preparations with dual action, antihistamine and mast cell-stabilizing effects, providing simultaneous histamine receptor antagonist effects, stabilizing mast cell membranes and modifying the action of eosinophils.67 Most ocular allergy patients concomitantly suffer from systemic symptoms and although second-generation oral antihistamines are preferred due to their fewer adverse systemic side effects, they induce ocular drying.68
Steroids are the most potent medications used in allergic conjunctivitis and are effective in treating both acute and chronic presentations.69 Yet, as with any medication, there are limitations with steroid use, and a short course of steroid therapy may be prudent. A low-dose, non–ketone-based steroid should be considered for the long term. Non-steroidal anti-inflammatory drugs can also be added to the treatment regimen as well as other steroid-sparing agents such as cyclosporin or cyclosporine A and tacrolimus in treating severe and chronic forms of ocular allergies.
Systemic Disease Association
Conjunctivitis may be the initial presentation for many systemic diseases. These can include reactive arthritis, manifesting as conjunctival hyperemia with purulent discharge (an essential component of Reiter’s triad); rosacea, including a follicular and papillary reaction, cicatrization of the conjunctiva and scarring secondary to entropion and trichiasis; and graft-vs.-host disease with conjunctival involvement indicating a more severe systemic involvement and poor prognosis.70-72 Ocular cicatricial pemphigoid, though rare, can induce loss of conjunctival goblet cells and drying of the ocular surface.73 Another example is Stevens-Johnson syndrome, which varies from conjunctival hyperemia to near-complete sloughing of palpebral conjunctiva and lid margins with acute ocular involvement reported in up to 88% of cases.74
Toxic Conjunctivitis
Long-term use of topical eye medications may induce ocular surface changes, including dry eye, conjunctival inflammation, ocular surface fibrosis and scarring.75 There is a high ocular morbidity seen in glaucoma patients as well as those who have undergone glaucoma surgery. Subclinical infiltration of the conjunctival epithelium and substantia propria by inflammatory cells has also been reported.76 Literature published during the past decade has pointed to the deleterious effects of benzalkonium chloride, which is often used as a preservative in eye drops, on the ocular surface.77 Limiting exposure to preservatives may diminish the toxic side effects of drops; this will likely lead to higher patient compliance and result in more favorable clinical outcomes, especially in those who need to be on glaucoma medications.
Takeaways
Conjunctivitis encompasses a wide range of diseases occurring worldwide. It rarely causes permanent vision loss, but its impact on patients’ quality of life can be considerable. Our clinical duty is to properly diagnose and, when necessary, treat this condition, whatever its origin, with a targeted approach.
Dr. Bloomenstein is the director of optometric service for the Schwartz Laser Eye Center in Scottsdale, AZ. Aside from contributing to numerous journals, he lectures frequently both nationally and internationally on various optometric topics. Dr. Bloomenstein has no financial interests pertaining to this article.
1. Weiner G. Demystifying the ocular herpes simplex virus. EyeNet. www.aao.org/eyenet/article/demystifying-ocular-herpes-simplex-virus. January 2013. 2.Shekhawat NS, Shtein RM, Blachley TS, et al. Antibiotic prescription fills for acute conjunctivitis among enrollees in a large United States managed care network. Ophthalmology. 2017;124:1099-1107. 3. de Laet C, Dionisi-Vici C, Leonard JV, et al. Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis. 2013;8:8-8. 4. Sati A, Sangwan VS, Basu S. Porphyria: varied ocular manifestations and management. BMJ Case Rep. 2013;2013:bcr2013009496. 5. Narayana S, McGee S. Bedside diagnosis of the ‘red eye’: a systematic review. Am J Med. 2015;128:1220-4.e1221. 6. Everitt H, Little P. How do GPs diagnose and manage acute infective conjunctivitis? A GP survey. Fam Pract. 2002;19:658-60. 7. Ryder E, Benson S. Conjunctivitis. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. 8. Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans Royal Soc Trop Med Hygiene. 1992;86:317-20. 9. Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmol. 2012;90:399-407. 10. Rietveld RP, van Weert HC, ter Riet G, et al. Diagnostic impact of signs and symptoms in acute infectious conjunctivitis: systematic literature search. BMJ. 2003;327:789. 11. Rietveld RP, ter Riet G, Bindels PJ, et al. Predicting bacterial cause in infectious conjunctivitis: cohort study on informativeness of combinations of signs and symptoms. BMJ. 2004;329:206-10. 12. Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis. Br J Gen Practice. 2005;55(521):962-4. 13. Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310:1721-9. 14. Kam KY, Ong HS, Bunce C, et al. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: a diagnostic accuracy study. Br J Ophthalmol. 2015;99:1186-9. 15. O’Brien T, Jeng B, McDonald M, et al. Acute conjunctivitis: truth and misconceptions. Curr Med Reg Opin. 2009;25(8):1953-61. 16. Stenson S, Newman R, Fedukowicz H. Laboratory studies in acute conjunctivitis. Arch Ophthalmol. 1982;100:1275-7. 17. Fitch CP, Rapoza PA, Owens S, et al. Epidemiology and diagnosis of acute conjunctivitis at an inner-city hospital. Ophthalmology. 1989;96:1215-20. 18. Rubenstein J, Spektor T. Conjunctivitis: infectious and noninfectious. In: Yanoff M, Duker JS, eds. Ophthalmology. 5th ed. Philadelphia: Elsevier; 2019:183-91. 19. Li J, Lu X, Sun Y, et al. A swimming pool-associated outbreak of pharyngoconjunctival fever caused by human adenovirus type 4 in Beijing, China. Int J Infect Dis. 2018;75:89-91. 20. Harley D, Harrower B, Lyon M, et al. A primary school outbreak of pharyngoconjunctival fever caused by adenovirus type 3. Commun Dis Intell. 2001;25:9-12. [PubMed] 21. Sinclair RG, Jones EL, Gerba CP. Viruses in recreational water-borne disease outbreaks: a review. J Appl Microbiol. 2009;107:1769-80. 22. Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. Adenoviral keratoconjunctivitis. Surv Ophthalmol. 2015;60:435-43. 23. Chintakuntlawar AV, Chodosh J. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul Immunol Inflamm. 2010;18:341-5. 24. Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist. 2018;11:981-93. 25. Richmond S, Burman R, Crosdale E, et al. A large outbreak of keratoconjunctivitis due to adenovirus type 8. J Hyg (Lond). 1984;93:285-91. 26. Okumus S, Coskun E, Tatar MG, et al. Cyclosporine a 0.05% eye drops for the treatment of subepithelial infiltrates after epidemic keratoconjunctivitis. BMC Ophthalmol. 2012;12:42. 27. Ghanem RC, Vargas JF, Ghanem VC. Tacrolimus for the treatment of subepithelial infiltrates resistant to topical steroids after adenoviral keratoconjunctivitis. Cornea. 2014;33:1210-3. 28. Gallenga PE, Lobefalo L, Colangelo L, et al. Topical lomefloxacin 0.3% twice daily versus tobramycin 0.3% in acute bacterial conjunctivitis: a multicenter double-blind phase III study. Ophthalmologica. 1999;213:250-7. 29. Jackson WB, Low DE, Dattani D, et al. Treatment of acute bacterial conjunctivitis: 1% fusidic acid viscous drops vs. 0.3% tobramycin drops. Can J Ophthalmol. 2002;37:228-37; discussion 237. 30. Varu DM, Rhee MK, Akpek EK, et al. Conjunctivitis preferred practice patternⓇ. Ophthalmology. 2019;126:P94-P169. 31. Tabbara KF, Jarade EF. Ganciclovir effects in adenoviral keratoconjunctivitis [abstract 3111-B253] ARVO. 2001;42:S579. 32. Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. Am J Med Sci. 2008;336:254-64. 33. Trinavarat A, Atchaneeyasakul LO. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: a pilot study. J Ocul Pharmacol Ther. 2012;28:53-8. 34. Kuo IC. Adenoviral keratoconjunctivitis: diagnosis, management, and prevention. Curr Ophthalmol Rep. 2019;7:118-27. 36. Sheikh A, Hurwitz B, van Schayck CP, et al. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2012;19:Cd001211. 37. Puri LR, Shrestha GB, Shah DN, et al. Ocular manifestations in herpes zoster ophthalmicus. Nepal J Ophthalmol. 2011;3:165-71. 38. Epling J. Bacterial conjunctivitis. BMJ Clin Evid. 2012;2012:0704. 39. Hovding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5-17. 40. Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet. 2005;366:37-43. 41. Schiebel NE. Evidence-based emergency medicine/systematic review abstract. Use of antibiotics in patients with acute bacterial conjunctivitis. Ann Emer Med. 2003;41:407-9. 42. Horven I. Acute conjunctivitis. A comparison of fusidic acid viscous eye drops and chloramphenicol. Acta Ophthalmol. 1993;71:165-8. 43. Shanmuganathan VA, Armstrong M, Buller A, et al. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye (Lond). 2005;19:284-91. 44. Gross RD, Lichtenstein S, Schlech BA, et al. Early clinical and microbiological responses in the treatment of bacterial conjunctivitis with moxifloxacin ophthalmic solution 0.5% (Vigamox����) using B.I.D. dosing. Today Ther Trends. 2003;21:227-37. 45. Granet DB, Dorfman M, Stroman D, et al. A multicenter comparison of polymyxin B sulfate/trimethoprim ophthalmic solution and moxifloxacin in the speed of clinical efficacy for the treatment of bacterial conjunctivitis. J Pediatr Ophthalmol Strabismus. 2008;45:340-9. 46. Leibowitz HM. The red eye. New Engl J Med. 2000;343:345-51. 47. Tabbara KF, El-Sheikh HF, Islam SM, et al. Treatment of acute bacterial conjunctivitis with topical lomefloxacin 0.3% compared to topical ofloxacin 0.3%. Eur J Ophthalmol. 1999;9:269-75. 48. Abelson MB, Heller W, Shapiro AM, et al. Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am J Ophthalmol. 2008;145:959-65. 49. Shields T, Sloane PD. A comparison of eye problems in primary care and ophthalmology practices. Fam Med. 1991;23:544-6. 50. Costumbrado J, Ng DK, Ghassemzadeh S. Gonococcal conjunctivitis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. 51. Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153(Suppl 1):17-21. 52. Bonini S. Atopic keratoconjunctivitis. Allergy. 2004;59:71-3. 53. Friedlander M. Ocular allergy. Curr Opin Allergy Clin Immunol. 2011;11(5):477-82. 54. Rathi VM, Murthy SI. Allergic conjunctivitis. Commun Eye Health. 2017;30:S7-S10. 55. Sofi RA, Mufti A. Vernal keratoconjunctivitis in Kashmir: a temperate zone. Int Ophthalmol. 2016;36:875-9. 56. De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013;97:9-14. 57. Solomon A. Corneal complications of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2015;15:489-94. 58. Totan Y, Hepsen IF, Cekic O, et al. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: a videokeratographic study. Ophthalmology. 2001;108:824-7. 59. Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-85. 60. Offiah I, Calder VL. Immune mechanisms in allergic eye diseases: what is new? Curr Opin Allergy Clin Immunol. 2009;9:477-81. 61. Li A, Li S, Ruan F, et al. Atopic keratoconjunctivitis: a diagnostic dilemma-a case report. Medicine. 2018;97:e0372. 62. Vengayil S, Vanathi M, Dada T, et al. Filtering bleb-induced giant papillary conjunctivitis. Cont Lens Anterior Eye. 2008;31:41-3. 63. Forister JF, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009;35:176-80. 64. Donshik PC, Ehlers WH, Ballow M. Giant papillary conjunctivitis. Immunol Allergy Clin North Am. 2008;28:83-103, vi. 65. Schmid KL, Schmid LM. Ocular allergy: causes and therapeutic options. Clin Exp Optom. 2000;83:257-70. 66. Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis: systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-6. 67. Mishra GP, Tamboli V, Jwala J, et al. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. 2011;5:26-36. 68. Welch D, Ousler GW, 3rd, Nally LA, et al. Ocular drying associated with oral antihistamines (loratadine) in the normal population-an evaluation of exaggerated dose effect. Adv Exp Med Biol. 2002;506:1051-5. 69. Pflugfelder S, Maskin S, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension 0.5% and placebo for the treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444-57. 70. Faraj HG, Hoang-Xuan T. Chronic cicatrizing conjunctivitis. Curr Opin Ophthalmol. 2001;12:250-7. 71. Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation. III. Conjunctival graft-vs-host disease. Arch Ophthalmol. 1989;107:1343-8. 72. Azari A, Rezaei Kanavi M, Potter H, et al. Autologous serum eye drop is safe and effective for treatment of dry eyes in graft-versus-host disease. Invest Ophthalmol Vis Sci. 2013;54:4330. 73. Wang K, Seitzman G, Gonzales JA. Ocular cicatricial pemphigoid. Curr Opin Ophthalmol. 2018;29:543-51. 74. Kohanim S, Palioura S, Saeed HN, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. 2016;14:168-88. 75. Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993;77:590-6. 76. Mietz H, Niesen U, Krieglstein GK. The effect of preservatives and antiglaucomatous medication on the histopathology of the conjunctiva. Graefes Arch Clin Exp Ophthalmol. 1994;232:561-5. 77. Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312-34. |
