Arm Yourself for Dry AMD
It’s imperative we prepare ourselves with up-to-date information for better care of our patients.
By Andrew J. Rixon, OD, Richard C. Trevino, OD, and Roya Attar, OD
Release Date:
January 2017
Expiration Date:
January 15, 2020
Goal Statement:
Proper AMD detection, education and management is essential, and staying current on information surrounding the disease allows for best patient outcomes. This article reviews the pathophysiology, risk factors, diagnostics and patient follow up to ensure clinicians are ready for these patients.
Faculty/Editorial Board:
Andrew Rixon, OD, Richard Trevino, OD, Roya Attar, OD
Credit Statement:
This course is COPE approved for 2 hours of CE. Course ID is 51948-PS. Check with your local state licensing board to see if this counts toward your requirement for relicensure.
Disclosure Statement:
The authors have no relationships todisclose.
Age-related macular degeneration (AMD) is the leading cause of vision loss in individuals 65 and older.1 By the year 2020, it will affect an estimated 196 million people worldwide.1 Vision loss from AMD can be functionally and emotionally debilitating, as it can make it difficult, or even impossible, to read, drive, enjoy certain hobbies and maintain an independent lifestyle.2
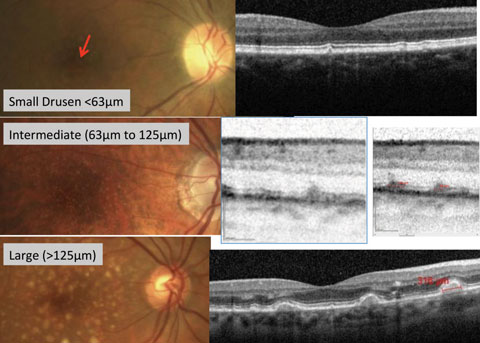 |
| Fig. 1. Fundus photography with corresponding OCT images of small, intermediate and large drusen. |
Approximately 90% of those with AMD have the dry form, for which there is currently no treatment—only lifestyle modifications to reduce risk of progression. Roughly 10% of those with AMD develop choroidal neovascular membranes (CNV), yet it accounts for 75% of severe vision loss in those with AMD.3
The capacity for proper detection, education and management of AMD is essential for optometrists, and staying current on the ever-changing body of information surrounding the disease allows for best patient outcomes. This article reviews the pathophysiology of dry AMD, risk factors, diagnostics and patient follow up to ensure clinicians are ready when patients present with suspicious findings. While there is no treatment as of yet, review of current clinical trials suggests one might await us in the future.
Pathophysiology
Retinal health is contingent on the relationship between photoreceptors and the retinal pigment epithelium (RPE).4 The RPE functions as a protector against photo-oxidative damage to the retina and transports nutrients between the choriocapillaris and retina.4 To avoid photo-oxidative damage, photoreceptors undergo a daily renewal process where roughly 10% of their volume is shed, then phagocytosed by the RPE.4
Foundationally, the accumulation of photo-oxidized debris within and under the RPE is considered the initiating cause of AMD.5 The debris found within the RPE cells includes a yellow-brownish pigment granule called lipofuscin—a lipid-containing residue from lysosomal digestion with autofluorescent properties.6
Drusen, composed of acellular, polymorphous material, is considered the hallmark of early AMD.7 Hard drusen involve focal thickening of the RPE basement membrane and may become calcified, lipidized, cholesterolized or, rarely, vacscularized.8 Soft drusen are substantially larger than hard drusen and represent a limited separation of the RPE basement membrane from its attachment to Bruch’s membrane.8
Drusen size and RPE abnormalities are important risk factors for progression of AMD (Figure 1).9 RPE cells, in response to many negative stimuli, go through morphological changes such as hypertrophy, atrophy and intraretinal migration.7
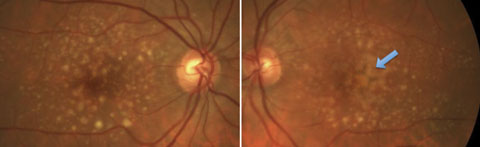 |
| Fig. 2. Both large drusen and pigmentary abnormalities in a patient with a high risk for conversion to advanced AMD. |
Small drusen (<63µm) are consistent with the normal aging process and have no relevant increased risk of late AMD developing.10 In fact, small drusen (<31.5µm) are common in persons younger than 50, with reported incidence as high as 95.5%.3,11,12
Medium drusen (63µm to 125µm) have not been studied extensively, but a recent study found that patients with large total macular areas involving medium drusen, and closer proximity of these to the fovea, were more likely to progress to early AMD.13 Medium drusen confers an increased risk of progression to late AMD, although this risk is not great.10,13 However, the presence of both medium drusen and RPE abnormalities (within two disc diameters of the fovea) increases the risk of progression to late AMD by between four and ten-fold compared with the presence of medium drusen alone.10,13
Large drusen (>125µm) are associated with a much higher risk for developing advanced AMD, with an estimated five-year rate of developing late AMD of 13% when found bilaterally without other abnormalities.10,9 That five-year risk of progression increases to 47.3% when there is the bilateral presence of both large soft drusen and pigmentary abnormalities (Figure 2).10
The width of a major branch retinal vein as it crosses the optic disc margin is approximately 125µm and is a good reference to estimate the size of retinal drusen.10
 |
| Fig. 3a. Radial OCT slice showing spontaneous regression of large drusen. |
 |
| Fig. 3b. Radial slice in same patient, in same time frame, showing increased size and formation of large drusen. |
The natural history of drusen is dynamic, and multiple studies confirm that both resorption of drusen and the formation of new drusen can occur simultaneously in the same macula.9,14 Recent preliminary SD-OCT studies show a tendency for drusen to increase in volume and area over time, although regression can also occur (Figures 3a and 3b).9,15 In the observation group of one study, the proportion of eyes showing a reduction of ≥50% in the area of drusen within 3,000µm of the foveal center increased over time from 1.2% at six months to 31.2% at five years.16
Although regression of drusen volume may seem to be a positive outcome, this usually progresses to outer retinal atrophy and loss of underlying choroidal thickness.17 Investigators found that larger drusen volume is more likely to spontaneously regress, followed by possible progression to geographic atrophy (GA) or CNV.9 Additional studies show that increased drusen volume with spontaneous regression is a negative prognostic indicator for advancement of the disease.18,19
Reticular pseudodrusen (RPD) has recently been recognized as another expression of AMD. RPD are associated with changes internal to the RPE and are predominantly located outside the fovea. RPD is highly correlated with GA, a known risk factor for advanced AMD.20 Approximately 30% to 50% of patients with RPD progress to late AMD.21 In the Beaver Dam eye study, patients with RPD had a six-fold higher rate of progression to late AMD than patients with indistinct soft drusen alone.22
Geographic atrophy occurs when the RPE, overlying photoreceptors and underlying choriocapillaris break down in a sharply demarcated area, revealing underlying choroidal vessels.22-25 Research estimates it accounts for 35% to 40% of late-stage AMD cases.26 GA develops frequently in macular areas previously occupied by drusen.24 Once GA develops, the atrophic area typically enlarges slowly and in a non-central location, ultimately involving the central macula and resulting in vision loss.23,27,28
AMD Risk Factors
Some risk factors for AMD are modifiable, while others are not:
Non-modifiable Risk Factors
Age. The most important risk factor for AMD is age itself. The prevalence of AMD among 60-year-olds is 0.9%.26,29 At age 70 it rises to 2.8%, and among those older than 80 the prevalence jumps to over 10%.26,29 Although the reason for this strong association is not clearly understood, both local retinal and broader systemic age-related changes are believed to play a role.
Ethnicity. Research suggests the prevalence of AMD varies widely among racial and ethnic groups. In North America, studies estimate AMD is twice as prevalent among Caucasians compared with African Americans, while late-stage AMD is roughly 10 times more prevalent among Caucasians than African Americans.29,30
Genetic factors. Close relatives of people with AMD are at an increased risk for the condition.31 Studies of twins reveal that the heritability of AMD ranges between 46% and 71%, with severe AMD being more heritable than the mild form of the disease.32
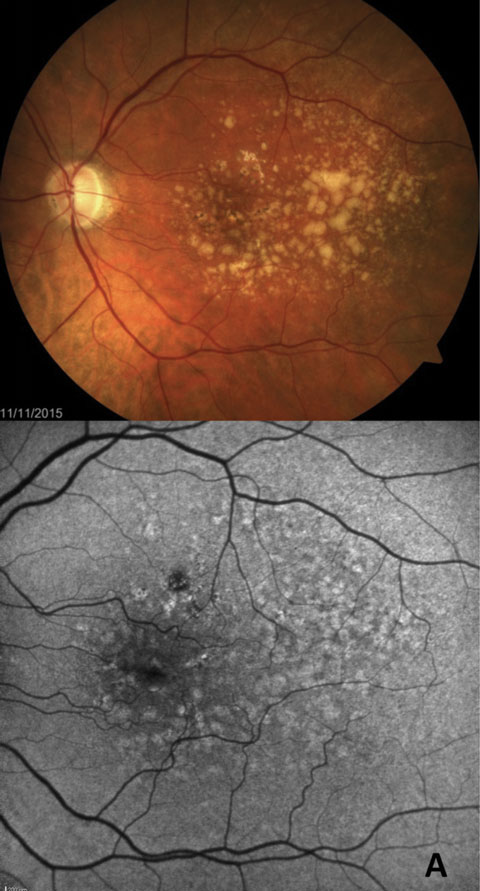
 |
 |
| Fig. 4. Comparison of fundus photography and FAF in GA progression over time. The top FAF and fundus photo are from 2009, the bottom are from 2012. |
Currently, 52 genes have been identified involved with AMD risk. Two genes in particular seem to convey the greatest risk.33 One is for a protein in the complement inflammatory pathway known as complement factor H (CFH). The second gene remains elusive, but studies have narrowed it down to either age-related maculopathy susceptibility gene number 2 (ARMS2) or a gene that codes for the protein high temperature requirement factor A1 (HTRA1), which plays a role in angiogenesis.34
Both CFH and ARMS2 were associated with higher rates of disease progression in the mostly white participants of the AREDS study, but the effect of these genes may vary with race.35 For example, it appears ARMS2 has little or no effect on AMD risk in African Americans.36 The risk associated with these two genes is additive, so a person with both high-risk genes is at greatest risk.37 Individuals possessing high-risk genes are not only more susceptible to developing AMD, but are also at elevated risk of having the disease progress to legal blindness.
Low macular pigment. This is another important AMD risk factor.38 Macular pigment is composed of the carotenoids lutein, zeaxanthin and meso-zeaxanthin, which have both blue light filtering and antioxidant properties. Low macular pigment is associated with low dietary intake of foods rich in these compounds such as spinach, kale and eggs. Other factors contributing to low macular pigment include genetics, obesity and smoking.39
High macular pigment optical density (MPOD), found using heterochromatic flicker photometry, is believed to protect the retina against photo-oxidative damage caused by blue light.38 Individuals with low MPOD are at elevated risk of AMD, and may also suffer from decreased visual function owing to the blue light filtering effect of macular pigment.38
Modifiable Risk Factors
Tobacco. By far the most important modifiable risk factor for AMD is smoking tobacco products, and it remains the only established causative factor for AMD.40
Compared with someone who has never smoked, current smokers have two to three times greater risk of developing the disease.41 In addition, smokers develop AMD at a younger age than nonsmokers and have a higher risk of disease progression.42
Smoking cessation results in a risk reduction that increases with duration of abstinence from tobacco. Several long-term, population-based studies found that former smokers are at only slightly higher risk than individuals who have never smoked.40
Lifestyle. In addition to smoking cessation, a number of other lifestyle changes can decrease AMD risk, including maintaining an average body weight, getting regular exercise and eating a heart-healthy diet.43 In fact, a combination of healthy lifestyle practices might be more important in reducing AMD risk than a focus on any given one. A healthy lifestyle can reduce oxidative stress and inflammation throughout the body, both of which are thought to promote AMD.
 |
| Fig. 5a. FAF more easily highlights early GA formation that is less detectable in fundus examination. |
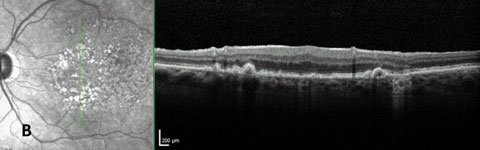 |
| Fig. 5b. GA confirmed with OCT showing increased transmission through to the choroid in region of GA. |
Increased exposure to sunlight. This has been identified as a potential AMD risk factor, and it appears to be greatest in those with light hair and eye color.44,45 Known as the blue light hazard, short wavelength, high energy, visible blue light triggers the release of harmful free radicals in the retina that cause oxidative stress, possibly contributing to the development of AMD.46 Measures that protect the eyes from sunlight, including broad-brimmed hats and blue light filtering sunglasses, can mitigate this risk.47 The yellowing of the crystalline lens with age will naturally decrease the amount of blue light that reaches the retina as a patient ages. However, cataract surgery can eliminate this protective effect, thereby increasing AMD risk.41 Many intraocular lens implants now contain blue light filtering properties. The AREDS study found no association between cataract surgery and subsequent progression of AMD.48
Associated Findings
Dark adaptation. In addition to a variety of visual changes, self-reported complaints of difficulty under dim lighting or at night are common in patients with AMD.49 Consistent with this complaint, delayed rod-mediated dark adaptation is characteristic of early AMD, which can also be observed in some older adults with normal macular health, whereas cone-mediated dark adaptation in the same retinal area is undisturbed.50
In early AMD, photoreceptor degeneration is associated with decreased light sensitivity in the macula and slowed dark adaptation, despite relatively unimpaired visual acuity.51 Research suggests this is likely due to changes within the RPE/Bruch’s membrane complex, where drusen are formed.49,51 Drusen accumulate in the aging Bruch's membrane and in the sub-RPE space in early AMD, disrupting the retinoid cycle and leading to photoreceptor degeneration, with an earlier onset and more severity affecting macular region rods than the cones.50,52 Other potential factors contributing to the scotopic dysfunction in early AMD include genetic alterations in vitamin A metabolism and age- and disease-related deficits in pathways within the RPE metabolism that remain uncharacterized. Regardless of the exact mechanisms underlying the slowed rod-mediated dark adaptation, a scotopic functional impairment is present in the earliest phases of AMD.50
One study concluded that delayed rod-mediated dark adaptation in older adults with normal macular health is associated with incident-early AMD three years later, and thus is a functional biomarker for detection of early disease.53
Research has also found increasing age, decreasing visual acuity, the presence of reticular pseudodrusen, severity of AMD and decreased subfoveal choroidal thickness are also associated with dark adaptation impairments.54
Imaging in Dry AMD
Standard color fundus photography, although historically useful for classifying the stage of AMD, may not adequately detect some common early and intermediate manifestations.55,56 RPE damage is a hallmark of AMD, and alterations to the RPE may not be clinically detectable by funduscopy or photography. Drusen and subretinal drusenoid deposits become clinically visible at 30µm while changes in RPE cells are substantially smaller.57 In vivoimaging of the autofluorescent properties of the ocular fundus provides the ability to visualize and evaluate the state of the aging RPE in AMD.58
Studies show innate autofluorescent properties originate from the accumulation of fluorescent pigments, known as fluorophores, in the RPE cells, primarily as lipofuscin.58 Fundus autofluorescence (FAF) noninvasively visualizes these fluorophores with a short wavelength excitation light, followed by capture of the fluorescence signals emitted post excitation.59 Areas of abnormal autofluorescence are then compared with a normal, homogenous autofluorescent background and are described as having increased or decreased autofluorescence.60 GA, for instance, exhibits dark areas because of a complete lack of fluorophores.60 FAF thus provides a topographical rendering of the extent of lipofuscin accumulation in the RPE.8
Using these renderings, researchers have described and classified distinct patterns of abnormal fundus autofluorescence in early nonexudative AMD—and, most importantly, suggest they are useful in determining the risk of disease progression.58,59,61 Furthermore, researchers have used FAF to demonstrate not only progression in patients with GA, but also inhibition of progression when therapeutic intervention is successful (Figure 4).59,62,63 A recent study shows that FAF imaging detects GA earlier than with color photography, in part due to precise delineation of GA borders as a result of superior contrast (Figures 5a and 5b).28 However, FAF’s advantage over color photography diminishes over time, with the two modalities ultimately becoming comparable in more advanced cases.64
FAF is also highly beneficial in imaging RPD. The appearance of these drusen vary based on imaging techniques, and their extent of involvement can be difficult to detect with fundus evaluation alone.20 These lesions tend to show well on FAF and infrared reflectance imaging (Figure 6).20
OCT has become increasingly valuable in AMD assessment, as it provides noninvasive, high-resolution, cross-sectional imaging of both the neurosensory and deeper subretinal layers.66 SD-OCT has proven useful for evaluating drusen of all sizes, drusenoid PEDs, changes to neurosensory retina overlying drusen, reticular pseudodrusen, retinal pigment abnormalities, GA and age-related choroidal atrophy.7
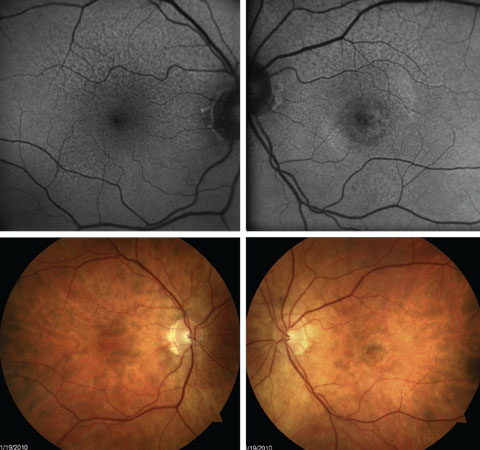 |
| Fig. 6. FAF shows greater extent of RPE abnormality with extrafoveal RPD than is easily seen with fundus photography. This patient also has presence of central CNV OS. |
Specifically, small to medium drusen will exhibit variable reflectivity depending on the composition of the underlying material. Large drusen or drusenoid PEDs will often show a dome-shaped elevation of the RPE with a hypo- or medium-reflective material separating the RPE from the underlying Bruch’s membrane.7 Pigment clumping and migration will appear focally hyper-reflective with underlying shadowing (Figure 7). Focal loss of RPE will show hyporeflectivity in the RPE and hyper-reflectivity of the underlying choroidal vessels.7 Lastly, GA appears as areas of sharply demarcated choroidal hyper-reflectivity. There may be associated retinal atrophy manifesting with thinning or loss of the outer nuclear layer and the absence of the external limiting membrane and inner segment-outer segment junctions (Figure 8).7
Current Clinical Trials
Currently, medical treatment options for AMD are limited to only patients whose disease leads to the development of CNV. With anti-vascular endothelial growth factor (VEGF) treatment for these particular patients, the visual prognosis for exudative AMD has improved drastically, but investigators are still evaluating multiple targets and different delivery systems to further improve treatment for those with CNV from AMD. Additionally, researchers are making significant progress in helping dry AMD patients who suffer vision loss from GA.
One goal for future AMD treatment is improving treatment efficacy by targeting multiple steps simultaneously in the pathogenesis of CNV development. Two drug targets researchers are currently considering are angiopoietin 2 and platelet-derived growth factor (PDGF), as both play key roles in the formation of new blood vessels.66 Investigators are also evaluating the molecule RG7716 in phase II clinical trials of the AVENUE study.67 It is an anti-VEGF molecule, but also exhibits anti-angiopoietin 2 properties.68 Fovista (Ophthotech) is an anti-PDGF molecule that has completed phase II trials, and preliminary results show increased efficacy when used in conjunction with ranibizumab compared with ranibizumab alone.69,70 It is currently in phase III clinical trials.71,72
Another emphasis in AMD therapy is relieving patient’s burden of treatment. Although current anti-VEGF therapy provides extreme improvement in visual outcomes for those with wet AMD, many patients maintain visual stability only with periodic injections, often monthly, for an indefinite length of time. The ongoing LADDER study is evaluating the feasibility of a port delivery system to give sustained release of medication in those with wet AMD.73 Additionally, the phase III clinical trial HAWK is evaluating the efficacy of an anti-VEGF agent, RTH258, that could decrease the time between retreatment in patients with CNV.74
RTH258 is currently the smallest VEGF inhibitor used in human therapy. Due to its small molecular size, it can be given in higher concentrations, hopefully leading to longer duration of action. In initial phase II studies, researchers show it is non-inferior to ranibizumab one-month post treatment and had longer effect of treatment than ranibizumab.75
 |
The most promising treatment options on the horizon for GA are complement inhibitors, which aim to decrease the rate of progression of GA. Phase II clinical trials with intravitreal dosing show lampalizumab, a complement factor D inhibitor, is a safe treatment option for GA and has potential efficacy in reduction of GA progression at 18 months.76 Currently there are two ongoing identical phase III trials, CHROMA and SPECTRI, to determine lampalizumab’s efficacy.77,78
 |
| Fig. 7. Pigmentary migration imaged with OCT shows increased hyper-reflectivity (darker with reverse contrast scan) and causes shadowing of underlying RPE layer. |
 |
| Fig. 8. OCT image of GA shows increased light transmission to the choroid due to absence of RPE. The outer retinal layers are also lost in the regions of GA. |
While lampalizumab is a promising treatment option, other complement inhibitors have failed to show efficacy. For example, eculizumab, a factor C5 inhibitor, failed to show efficacy in reduction of GA progression in phase III clinical trials.79
With rising incidence of AMD in the aging US population, optometrists will have to assess and manage more patients afflicted with this potentially debilitating condition. We must stay abreast of current and upcoming means to diagnose and manage AMD. For example, technological advances in ocular imaging are allowing for quicker detection of small drusen and RPE abnormalities, earlier detection of GA, and improved visualization of retinal structure that was previously unobservable with funduscopy alone.
Additionally, we are often faced with family members seeking answers to their questions and concerns, and we owe it to them to address their trepidations with accurate information on current and future treatment options. We can only serve the best interest of our patients by arming ourselves with the knowledge and skill necessary to manage dry AMD in this ever-evolving landscape.
Dr. Rixon practices at the Memphis VA medical center, where he is the residency coordinator.
Dr. Trevino is an associate professor at the Rosenberg School of Optometry, where he serves as director of Residency Programs and chief of the Ocular Health Service.
Dr. Attar is the director of professional relations and an assistant clinical professor at the University of Pikeville - Kentucky College of Optometry (KYCO).
The authors would like to thank Mohammad Rafieetary, OD, and Jessica Haynes, OD, Optometric Retina Fellow, both at the Charles Retinal Institute, for contributing their expertise to this article.
|
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet. Global health. 2014;2(2):e106–16. 2. Williams RA. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998;116(4):514. 3. Klein R, Klein EKB, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7-21. 4. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photorecptors. Physiology (Bethesda). 2010;25(1):8-15. 5. Kijlstra A, Berendschot TT. Age related macular degeneration: a complementopathy? Ophthalmic Res. 2015;54(2):64-73. 6. Algvere PV, Kvanta A, Seregard S. Drusen maculopathy: a risk factor for visual deterioration. Acta Ophthalmol. 2016:94(5):427-33. 7. Keane PA, Patel PJ, Liakopoulos S. Evaluation of age related macular degeneration with optical coherence tomography. Surv Ophthalmol. 2012:57(5):389-414. 8. Zajac-Pytrus HM, Pilecka A, Turno-Krecicka A. The dry form of age related macular (AMD) degeneration: the current concepts of pathogenesis and prospects for treatment. Adv Clin Exp Med. 2015;24(6):1099-1104. 9. Yehoshua Z, Wang F, Rosenfeld PJ, et al. Natural history of drusen morphology in age related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12):2434-41. 10. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age related macular degeneration. Ophthalmol. 2013;120(4):844-51. 11. Silvestri G, Williams MA, McAuley C, et al. Drusen prevalence and pigmentary changes in Caucasians aged 18-54 years. Eye (Lond). 2012;26(10):1357-62. 12. Munch IC, Sander B, Kessel L, et al. Heredity of small hard drusen in twins aged 20-46 years. Invest Ophthalmol Vis Sci. 2007;48:833-8. 13. Joachim N, Mitchell P, Kifley A, Wang JJ. Incidence, progression, and associated risk factors of medium drusen in age related macular degeneration: findings from the 15 year follow up an Australian cohort. JAMA Ophthalmol. 2015;133(6):698-705. 14. Sallo FB, Rechtman E, Peto T, et al. Functional aspects of drusen regression in age related macular degeneration. Br J Ophthalmol. 2009;93(10):1345-50. 15. Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age related macular degeneration. Ophthalmology. 2011;118(7):1373-9. 16. Complications of Age Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the complications of age related macular degeneration prevention trial. Ophthalmology. 2006;113(11):1974-86. 17. Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age related macular degeneration. Retina. 2013;33(9):1800-8. 18. Schlantz FG, Baumann B, Kundi M, et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. April 4, 2016. [Epub ahead of print]. 19. Folgar FA, Yuan EL, Sevilla MB, et al. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology. 2016;123(1):39-50. 20. Sivaprasad S, Bird A, Nitiahpapand R, et al. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016;61(5):521-37. 21. Joachim N, Mitchell P, Rochtchina E, et al. Incidence and progression of reticular drusen in age related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014;121(4):917-25. 22. Klein R, Meuer SM, Knudtson MD, et al. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145(2):317-26. 23. Brader HS, Ying GS, Martin ER, et al. Characteristics of incident geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2013;120(9):1871-9. 24. Joachim N, Mitchell P, Kifley A, et al. Incidence and progression of geographic atrophy: observations from a population based cohort. Ophthalmology. 2013;120(10):2042-50. 25. Chaikitmongkai V, Tadarati M, Bressler NM. Recent approaches to evaluating and monitoring geographic atrophy. Curr Opin Ophthalmol. 2016;27(3):217-23. 26. Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. 27. Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677-91. 28. Domalpally A, Danis R, Agron E, et al. Evaluation of geographic atrophy from color photographs and fundus autofluorescence images: age related eye disease study 2 report number 11. Ophthalmology. 2016;123(11):2401-7. 29. Klein R, Klein BEK. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Invest Ophthalmol Vis Sci. 2013;54:15-8. 30. Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106: 1049–55. 31. Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123:199–206. 32. Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123: 321–7. 33. Schwartz SG, Hampton BM, Kovach JL, Brantley MA. Genetics and age-related macular degeneration: a practical review for the clinician. Clin Ophthalmol. 2016;10:1229–35. 34. Zhang L, Lim SL, Du H, et al. High temperature requirement factor A1 (HTRA1) gene regulates angiogenesis through transforming growth factor-β family member growth differentiation factor 6. J Biol Chem. 2012;287:1520-6. 35. Seddon JM, Francis PJ, George S, et al. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297:1793–800. 36. Restrepo NA, Spencer KL, Goodloe R, et al. Genetic determinants of age-related macular degeneration in diverse populations from the PAGE study. Invest Ophthalmol Vis Sci. 2014;55:6839–50. 37. Rivera A, Fisher S, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. 38. Davies NP, Morland AB. Macular pigments: Their characteristics and putative role. Prog Retin Eye Res. 2004;23:533–59. 39. Nolan JM, Stack J, O’Donovan O, et al. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84:61–74. 40. Thornton J, Edwards R, Mitchell P, et al. Smoking and age-related macular degeneration: a review of association. Eye (Lond). 2005;19:935–44. 41. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. 42. Klein R, Knudtson MD, Cruickshanks KJ, Klein BEK. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:115–21. 43. Mares JA, Voland RP, Sondel SA, et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol. 2011;129:470-80. 44. Sui GY, Liu GC, Liu GY, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97:389–94. 45. Klein BEK, Howard KP, Iyengar SK, Sivakumaran T, et al. Sunlight exposure, pigmentation, and incident age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:5855–61. 46. Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23:523–31. 47. Tomany SC, Cruickshanks KJ, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2004;122:750–7. 48. Chew EY, Sperduto RD, Milton RC, et al. Risk of advanced age-related macular degeneration after cataract surgery in the Age-Related Eye Disease Study: AREDS report 25. Ophthalmology. 2009;116:297–303. 49. Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427–31. 50. Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health functioning in oler adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55(8):4776–89. 51. Sevilla MB, McGwin G,Jr Lad EM, et al. Relating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. American J Ophthalmol. 2016;165:65-77. 52. Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Research Review. 2002;1(3):381-96. 53. Owsley C, McGwin G Jr, Clark M, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123(2):344-51. 54. Flamendorf J, Agron E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122(10):2053-62. 55. Age related eye disease study research group. The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the age related eye disease study report number 6. Am J Ophthalmol. 2001;132(5):668–81. 56. Holz FG, Steinberg JS, Gobel A, et al. Fundus autofluorescence imaging in dry AMD: 2014 jules gonin lecture of the retina research foundation. Graefes Arch Clin Exp Ophthalmol. 2015;253(1):7-16. 57. Ach T, Tolstik E, Messinger JD, et al. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(5):3242–52. 58. Bindewald A, Bird AC, Dandekar SS. Classification of fundus autofluorescence patterns in early age related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46(9):3309-14. 59. Wu Z, Luu CD, Ayton LN, et al. Fundus autofluorescent characteristics of nascent geographic atrophy in age related degeneration. Invest Ophthalmol Vis Sci. 2015;12;56(3):1546-52. 60. von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38(2):478–86. 61. Smith T, Chan JK, Busuoic M, et al. Autofluorescence characteristics of early, atrophic and high risk fellow eyes in age related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(12):5495-55. 62. Pilotto E, Guidolin F, Convento E, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age related macular degeneration. Br J Ophthalmol. 2013;97(5):622–6. 63. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age related macular degeneration. Am J Ophthalmol. 2007;143:463–72. 64. Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, et al. Evaluation of autofluorescence imaging with the scanning laser ophthalmoscope and the fundus camera in age related geographic atrophy. Am J Ophthalmol. 2008;146:183-92. 65. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178-81. 66. Rubio RG, Adamis AP. Ocular angiogenesis: vascular endothelial growth factor and other factors. Developments in Ophthalmology Retinal Pharmacotherapeutics. 2016;55:28-37. 67. Hoffmann-La Roche. A multiple-center, multiple-dose and regimen, randomized, active comparator controlled, double-masked, parallel group, 36 week study to investigate the safety, tolerability, pharmacokinetics, and efficacy of RG7716 administered intravitreally in patients with choroidal neovascularization secondary to age-related macular degeneration. Available at https://clinicaltrials.gov/ct2/show/NCT02484690. Accessed October 31, 2016. 68. Klein C, Schaefer W, Regula JT. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs. 2016;8(6):1010-20. 69. Ophthotech. Fovista Comments. Available at www.ophthotech.com/product-candidates/fovista. Accessed October 31, 2016. 70. Ophthotech Corporation. An 18 month phase 2a open label, randomized study of Avastin, Lucentis, or Eylea (anti-VEGF therapy) administered in combination with Fovista (anti-PDGF BB pegylated aptamer). Available at https://clinicaltrials.gov/ct2/show/NCT02387957. Accessed September 26, 2016. 71. Ophthotech Corporation. A phase 3 randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous administration of Fovista (anti PDGF-B pegylated aptamer) administered in combination with Lucentis compared to Lucentis monotherapy in subjects with subfoveal neovascular age-related macular degeneration. Available at https://clinicaltrials.gov/ct2/show/NCT01944839. Accessed September 26, 2016. 72. Ophthotech Corporation. A phase 3 randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous administration of Fovista (anti PDGF-B pegylated aptamer) administered in combination with either Avastin or Eylea compared to Avastin or Eylea monotherapy in subjects with subfoveal neovascular age-related macular degeneration. Available at https://clinicaltrials.gov/ct2/show/NCT01940887. Accessed September 26, 2016. 73. Genentech. A phase II multicenter, randomized, active treatment-controlled study of the efficacy and safety of the ranibizumab port delivery system for sustained delivery of ranibizumab in patients with subfoveal neovascular age-related macular degeneration (LADDER). Available at https://clinicaltrials.gov/ct2/show/NCT02510794. Accessed September 26, 2016. 74. Alcon Research. Two-year, randomized, double-masked, multicenter, three-arm study comparing the efficacy and safety of RTH258 versus aflibercept in subjects with neovascular age-related macular degeneration. Available at https://clinicaltrials.gov/ct2/show/NCT02307682. Accessed September 26, 2016. 75. Holz FG, Dugel PU, Weissgerber G, et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration. Ophthalmology. 2016;123(5):1080-9. 76. Roche's lampalizumab phase II data shows benefit in patients with the advanced form of dry age-related macular degeneration. Available at www.roche.com/investors/updates/inv-update-2013-08-27.htm. Accessed October 31, 2016. 77. Hoffmann-La Roche. A study investigating the safety and efficacy of lampalizumab intravitreal injections in patients with geographic atrophy secondary to age-related macular degeneration (SPECTRI). Available at https://clinicaltrials.gov/ct2/show/NCT02247531. Accessed September 26, 2016. 78. Hoffmann-La Roche. A study investigating the efficacy and safety of lampalizumab intravitreal injections in participants with geographic atrophy secondary to age-related macular degeneration (CHROMA). Available at https://clinicaltrials.gov/ct2/show/NCT02247479NLM. Accessed September 26, 2016. 79. Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration. Ophthalmology. 2014;121(3):693-701. 80. American Optometric Association. Optometric clinical practice guideline. Care of the patient with age-related macular degeneration. Reference guide for clinicians. 2004. Available at www.aoa.org/documents/optometrists/CPG-6.pdf. Accessed September 26, 2016. 81. American Academy of Ophthalmology retina panel. Preferred Practice Pattern Guidelines. Age-related macular degeneration. San Francisco, CA: American Academy of Ophthalmology. 2008. Available at www.aao.org/ppp. Accessed September 26, 2016. |
