Are You Clear on Your Macular Function Screening Responsibilities?
How the latest technologies can change how you practice.
By Sherrol A. Reynolds, OD
Release Date:
April 2016
Expiration Date:
April 1, 2019
Goal Statement:
With the prevalence of macular disease rising, optometrists are more likely than ever to encounter damage to this structure. The many new imaging devices and functional tests available to evaluate the macula should be used in concert with each other by a skilled clinician. This article reviews the available tests, when—and for which patients—their use is appropriate and how their use has improved diagnostic capabilities for conditions such as vitreomacular adhesion, vitreomacular traction, hydroxychloroquine toxicity and others.
Faculty/Editorial Board:
Sherrol Reynolds, OD
Credit Statement:
COPE approval for 2 hours of CE credit is 49396-GO for this course. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement: This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Dr. Reynolds has no financial relationships to disclose.
Macular disease is on the rise in the United States. For example, advanced sight-threatening neovascular or “wet” age-related macular degeneration (AMD) is projected to increase to three million by 2020.1 Likewise, the spike in systemic conditions such as diabetes has led to a dramatic rise in diabetic retinopathy and maculopathy, which is projected to climb to 11 million by 2030, according the National Eye Institute.2
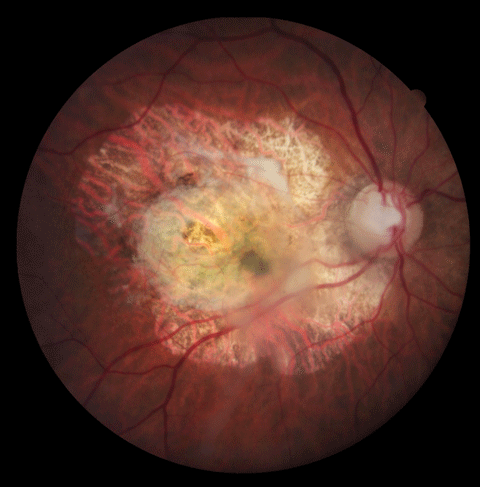 |
| Fundus photography is essential to documenting disease. For instance, this 85-year-old female patient’s advanced AMD is clearly visible. However, we now have a host of additional imaging capabilities that may enable earlier detection and intervention. |
Clinicians must provide patients timely detection, prompt management and, just as importantly, patient education to prevent vision loss. Macular function screening technologies are vital tools you’ll need to accomplish that.
This article provides an overview of those tools, what they can accomplish, the methods and techniques for evaluating macular function and guidelines for when to employ which technique.
Standard Macular Screening Procedures
In addition to visual acuity and color vision testing, basic procedures may be considered adjunctive screening techniques for the evaluation of macular function and disease. For example, the Watzke-Allen slit beam test provides subjective assessment in testing for a full-thickness macular hole defect. The central lens of the Goldmann three-mirror provides excellent stereoscopic views of subtle macular pathologies (i.e., cystoid macular edema, central serous chorioretinopathy). Amsler grid is beneficial in detecting metamorphopsia, central scotoma or micropsia. Although these procedures are subjective, they are noninvasive, readily accessible and provide early hints of macular abnormalities. Today, many optometrists have access to the following testing technologies as well:
• Fundus photography is essential in documenting and monitoring macular disease. It permits serial comparison of structural and functional changes as well as disease progression. High-quality fundus images can capture subtle early defects, such as exudates or microaneurysms, which can be missed during the clinical evaluation. It also has value as an educational tool for patients to learn about their condition—knowledge that may ultimately lead to better adherence. It also gives you exam findings in digital form to share with colleagues and other health providers.
Every practitioner should have access to a fundus camera. Combining that technology with other imaging modalities, such as spectral-domain optical coherence tomography (SD-OCT), is not only practical, but a sound return on the investment. Newer fundus cameras have the ability to adapt to smartphones, while others are available in combination with fundus autofluorescence (FAF) or optical coherence tomography (OCT) systems, allowing for multimodal imaging.
• SD-OCT provides high-resolution, volumetric and cross-sectional functional assessment and structural imaging of macular pathology. SD-OCT in particular is a must-have technology in the understanding, care and management of macular pathologies. Prior to SD-OCT, it would have been impossible to make an accurate diagnosis of vitreomacular adhesion (VMA) and vitreomacular traction (VMT). These devices enable early diagnosis and detection of an impending macular hole (MH). SD-OCT has greatly improved the potential for early detection of choroidal neovascular membrane in AMD, which has led to improved visual outcomes for many. In some cases, SD-OCT may be equal or superior to angiography in making the initial diagnosis (e.g., DME and CSCR).
OCT imaging has progressed to include newer imaging technology. For example, en face OCT imaging combines SD-OCT with transverse (C-scans) images of the macula.3 This allows for imaging individual retinal layers, especially in diseases that focally affect a specific retinal sublayer, such as AMD.
Newer enhanced-depth imaging (EDI) OCT provides improved visualization of the choroid, which allows for the assessment of choroidal changes in maculopathies such as AMD and polypoidal choroidal vasculopathies (PCV). While these imaging techniques may not be readily available in most optometric practices, patients who may benefit from the additional visualization could be comanaged with a retina specialist with access to EDI-OCT. In time, software upgrades to existing SD-OCT technology could make routine use of this modality possible.
| This SD-OCT image of a patient on Plaquenil (hydroxychloroquine-HCQ, Sanofi Aventis) for 15 years shows loss in photoreceptor inner/outer segment (IS/OS) junction and thinning of the outer retina in the parafoveal region. Click image to enlarge. |
Recently, the FDA approved optical coherence tomography angiography (OCTA), a noninvasive test that allows for the assessment of retinal and choroidal vasculature. Although fluorescein angiography (FA) and indocyanine green angiography (ICGA) remain commonly used for directing treatment of choroidal neovascularization (CNV) and retinal neovascularization, both are invasive tests with drawbacks, such as the potential for anaphylactic response.4-5 OCTA uses motion contrast—a 3D scanning technique—instead of intravenous dye to provide high-resolution images in seconds, which is an advantage over FA and ICGA, which take longer.6
Using OCTA, you can pinpoint the precise size and localization of lesions, visualize both the retinal and choroidal vasculature pattern and show structural and blood flow information. However, its disadvantages include a limited field of view and an inability to capture leakage.6
OCTA may not be readily available in most optometric practices; nevertheless, as the technology becomes available it has the potential to allow ODs enhanced evaluation of vascular changes like CNV in AMD and diabetic retinopathy.
• FAF imaging detects lipofuscin, a metabolic biomarker of the photoreceptor/retinal pigment epithelium (RPE) complex that indicates AMD, hereditary retinal disorders, toxic maculopathy and other macular diseases.7 FAF reveals RPE defects with reduced fluorescence and can show areas of photoreceptor damage—which appear as increased fluorescence from an accumulation of lipofuscin.8-9
• Automated 10-2 visual field testing is a valuable tool for functional assessment of macular damage. As the macula comprises ±8 degrees of the retina, using other tests, such as the 24-2 visual field, misses damage to this area. In some cases, AMD patients may retain good visual acuity, but experience distortion and other qualitative visual changes. Visual field assessment can detect the size and depth of the defects, leading to early detection and intervention of AMD. Likewise, a new Plaquenil (hydroxychloroquine-HCQ, Sanofi Aventis) screening guideline mandates a 10-2 white-stimulus visual field be performed on all patients taking this medication.
Additional Testing Options
Not all tests of macular function are readily available in the average OD’s office. Accessing many of these tests require connections with local retina specialists, eye hospitals or co-management with fellow ODs who have access to these technologies. Here are some of the less common, but still beneficial, technologies:
| This multispectral image reveals neovascular AMD with pigment epithelial detachments. |
• Macular pigment optical density (MPOD) testing measures macular pigments lutein and zeaxanthin, which help protect the photoreceptors from oxidative stress caused by ultraviolet and blue light damage.10 Low MPOD has been associated with potential progression of AMD.11
MPOD is a simple noninvasive test that uses heterochromatic flicker photometry technology to measure and gauge the effects of dietary change and supplementation of macular pigment; scores range from 0 to 1. Patients with low (0 to 0.21) or moderate (0.21 to 0.44) MPOD scores benefit from dietary changes and vitamin supplementation that increase their intake of the carotenoids lutein and zeaxanthin.12
The Age-Related Eye Disease Study (AREDS) showed a 25% beneficial effect of nutritional supplementation reducing the risk of progression to advanced AMD—at five years—in patients with intermediate AMD or with advanced AMD in one eye.13 Furthermore, AREDS2 demonstrated an 18% reduction in progression to advanced AMD with a recommended dose of 10mg lutein and 2mg of zeaxanthin for patients older than 50 years and with high risk for AMD progression.14
MPOD should be retested in three to six months, until the MPOD score registers 0.45 or above.
• Multispectral imaging (MSI) allows visualization of the retina in spectral slices, from the internal limiting membrane (ILM) to the choroid. MSI employs discrete, light-emitting wavelengths ranging from 520nm (green) to 940nm (infrared), which penetrate the choroidal layer.15
MSI technology provides a means to monitor RPE for changes or disease progression. Using MSI, you can detect various macular changes and conditions that “masquerade” as AMD, such as PCV. MSI is also available with FAF capability.
• Multifocal electroretinography (mfERG) creates a map of retinal function that reflects cone-mediated responses from the photoreceptor and bipolar cells.16 Eyes with macular disease, such as AMD and hereditary macular conditions, have reduced mfERG findings.17 This test is recommended for patients on Plaquenil therapy, as it can detect subtle changes in the early stages of toxicity. The most specific waveform pattern observed with Plaquenil toxicity is paracentral amplitude loss, indicative of decreased retinal function in the susceptible perifovea.18-19
• Dark adaptation provides automated functional assessment of dark adaptation time (AdaptDx, Maculogix). This automated test exposes a patient to bright light and plots a visual recovery curve. Dark adaptation has been shown to decrease in patients with early to late AMD and other conditions such as retinitis pigmentosa or inherited macular conditions.20
• Macular microperimetry allows for specific testing of macular function. It maps the pattern of a patient’s retinal sensitivity onto an image of that individual’s fundus. The advantage of microperimetry testing over a 10-2 visual field test is that it allows for the detection of small and discrete macular lesions and to retest these areas accurately over time.21 For example, in AMD, microperimetry can detect early functional changes for atrophy or neovascularization as well as monitor progression of the disease.
For Baby Boomers
In addition to the aforementioned technologies, additional measures can be employed as part of your macular screening responsibilities, leading to early detection or delayed progression for some common macular conditions.
AMD is on the rise, primarily among the rapidly aging Baby Boomer generation.22 Nearly 90% of patients have the “dry” or atrophic type of AMD, while 10% develop choroidal neovascularization (CNV), or “wet” AMD.23 Vision loss can occur if AMD is undetected, untreated, unsuccessfully treated or inappropriately treated.23
Assessing AMD risks is an important component in macular function and structural evaluation. For example, large drusen and retinal pigmentary abnormalities are established risks of AMD progression and functional loss. However, a recently published study found that patients are four times more likely to develop sight-threatening AMD if they show signs of medium drusen (63µm to 124µm) plus RPE abnormalities.24 Consequently, AMD patients with these findings require close monitoring for the early detection of a CNV, which requires prompt treatment with anti-VEGF.
Some AMD risks, such as smoking, obesity (BMI greater than 30) and cardiovascular risk (hypercholesterolemia and hypertension) can be changed.25 Light-induced (sunlight and blue light) oxidative damage can lead to the development of AMD and should be addressed with lenses designed to selectively filter or block UV or blue-violet light.26
Fixed factors such as race, gender (postmenopausal women are at greater risk), family history and genetics should be identified.27 The understanding of the role multiple genetic variants, specifically the complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) alleles, play in the development and progression of AMD has evolved. For example, complement factor genes CFH and C3, ARMS2 genes and other mitochondrial genes strongly suggest that inflammation contributes to the pathogenesis of AMD, so it may be worthwhile to assess genetic types using commercially available screening tools.28-29
Table 1. Differentiating VMT From VMA | ||
| IVTS Classification33 | MH classification | Management |
| VMA | Stage 0 | Observation |
| VMT with foveal detachment | Stage 1: macular cyst | Observation *3 months follow-up *Prompt RTC if new symptoms develop |
| A Stage 2 hole (<400µm) with 20/70 vision | FTMH with VMT | *Vitreoretinal surgery *Ocriplasmin |
| A Stage 3 hole (≥400µm) cuff of subretinal fluid VA 20/100 to 20/400 | FTMH with VMT | Vitreoretinal surgery |
| Stage 4 holes ((≥400μm) complete PVD 20/100 to 20/400 | FTMG without VMT | Vitreoretinal surgery |
VMA, VMT and MH together form a spectrum of disorders related to persistent vitreous adhesion at the vitreoretinal interface and anomalous or incomplete posterior vitreous detachment, common with aging.30 The adherent posterior vitreous cortex or posterior hyaloid membrane can exert tractional pull on the internal limiting membrane of the macula, resulting in the aforementioned conditions and potential visual loss.
OCT imaging is essential in understanding, visualizing and managing these conditions. VMA results from vitreous attachment to the macula, which can be focal (less than 1500µm) or broad (greater than or equal to 1500 µm) with no structural changes. VMT, either focal or broad, does result in structural changes—including distortion of the foveal surface, intraretinal pseudocyst and cystoid macular edema (CME). Now, our understanding of VMT has evolved to include an impending (stage 1) macular hole.31-33
These disorders may progress, remain stable or resolve spontaneously; therefore, it is imperative to re-examine patients using SD-OCT for persistent traction changes, including CME or a stage 1 MH (impeding MH), every six to 12 weeks, followed by every three months. Although some stage 1 macular holes may resolve spontaneously and completely, 50% may progress to a full-thickness macular hole (FTMH).34, 35 It’s important to assess the other eye as well, as 10% to 20% of patients develop a MH in the fellow eye, especially in the presence of VMA, within five years.36
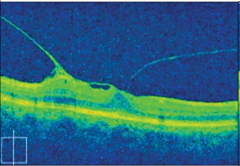 | 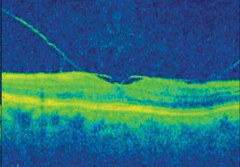 | |
| At left, VMT, and at right, VMA, are both conditions that would have been impossible to diagnose without the advent of OCT imaging. | ||
For Arthritis Patients
Plaquenil or the less frequently used Aralen (chloroquine-CQ, Sanofi Aventis) are anti-malarial medications prescribed for rheumatological disease such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). HCQ causes RPE degeneration with sparing of the foveal center, leading to bull’s eye maculopathy, due to its appearance.37 Anterior segment changes (such as corneal verticillata) should prompt careful structural and functional evaluation of the macula.
Risk for toxicity includes a dosage greater than 400mg/day, which is the commonly prescribed two 200mg pills per day. The patient’s height is also a risk, as those who are shorter than 5’7” in stature should take less than 400mg of Plaquenil per day.38 Because HCQ is not retained in fatty tissues, there is increased risk for obese patients (BMI greater than 30). Patients who are obese should be dosed on the basis of “ideal” body weight, which depends on the patient’s height, and not body weight. If this is not done, patients are at risk of being overdosed, thus increasing their risk of maculopathy. An optometrist’s vigilant watch over these patients and their dosing could help reduce their maculopathy risk.
| Macular damage from Plaquenil toxicity can be imaged using SD-OCT to look for the “flying saucer” sign. |
In addition to a standard dilated examination, the American Academy of Ophthalmology screening guideline recommends a white-stimuli 10-2 visual fields—which can detect subtle paracentral visual field defects, indicative of early toxic maculopathy—using either SD-OCT, FAF or mfERG to help monitor these patients.38
SD-OCT may detect significant structural alterations prior to the development of visible HCQ retinopathy, such as the loss of the external limiting membrane, disruption of the outer ellipsoid zone, parafoveal thinning of the outer nuclear layer and RPE damage.39 One notable finding is foveal sparing, sometimes referred to as the “flying saucer” sign of HCQ retinopathy.40 This ovoid appearance is created by the intact central foveal outer retinal structures contrasting to the adjacent perifoveal loss of the photoreceptor ellipsoid band and outer nuclear layer atrophy.40
Since the RPE is damaged in HCQ maculopathy, FAF intensity in the pericentral macula changes to a speckled or mottled appearance, which eventually merges into dark areas of absence of FAF signal.9
Table 2. HCQ Risk Factors | |
| Duration of Use | >5 years |
| Cumulative Dose | >1000g HCQ (7 years) |
| Daily Dose | >400mg (6.5mg/kg/day) HCQ |
| BMI (>30) Height (short) | HCQ is not retained in fat (adipose) tissue |
| Age | >60 years of age |
| Systemic Disease | Kidney or liver disease |
| Ocular Disease | Retinal or macular disease |
In using the functional mfERG test for HCQ toxicity suspects, look for paracentral amplitude loss, which is indicative of decreased retinal function in the susceptible perifoveal area.19
There is no consensus on which testing device is the best for detecting early HCQ toxicity. The take-away message is to not rely on any single procedure. The combined knowledge we can gather from not only structural but functional changes—via a combination of FAF, mfERG or SD-OCT—for concomitant abnormalities is imperative for comprehensive patient evaluation. Low-risk patients may be followed at five years, while those that are high risk should be evaluated annually.38
If probable or definite toxicity is detected, HCQ should be stopped immediately in consultation with the patient’s rheumatologist. Even then, those patients will continue to require close monitoring since progression of toxicity can endure for up to three years after discontinuing the medication.41,42
For Diabetes Patients
As diabetes has increased in prevalence over the last few decades, we have seen a corresponding dramatic rise in macular conditions such as diabetic macular edema (DME). Diabetic maculopathy is one of the main causes of poor visual functioning in patients with diabetes.43 The ETDRS deemed clinically significant DME—based on stereoscopic slit-lamp biomicroscopy and stereo color fundus photography—to include the following:44
1. Retinal thickening within 500µm of the macular center.
2. Hard exudates with thickening within 500µm of the macular center.
3. One or more disc diameters of retinal thickening, part of which is within one disc diameter of the macular center.
However, the EDTRS classification methods are subjective and may be unable to identify or localize small changes in retinal thickness, which can be observed on SD-OCT.45 OCT retinal thickness measurement is essential in monitoring progression and assessing treatment outcomes after laser photocoagulation, anti-VEGF and steroids or vitrectomy.46
| OCT image shows sponge-like swelling of the retina accompanied by intraretinal cystoid diabetic macular edema. |
Two major risk factors—disease duration and poor glycemic control—contribute considerably to the onset, severity and progression of complications. Other modifiable risk factors, including hypertension, dyslipidemia and obesity, should be assessed as well.47 Consequently, knowing the “ABCs” of diabetes—glycosylated hemoglobin (HbA1c), blood pressure (≤140/90) and cholesterol is key to DME assessment.
Some medications—for example Avandia (rosiglitazone, Glaxo SmithKline) and Actos (pioglitazone, Takeda Pharmaceuticals)—reportedly increase the risk of DME.48 So, you may have to be more vigilant in evaluating for DME among those patients taking these medications.
| OCT reveals bilateral perifoveal thinning with preservation of the central macula representing bull’s eye maculopathy. |
When patients experience visual symptoms-—even if they’re aware of a systemic disease—they may assume such changes stem simply from a refractive error. It is imperative to consider the possibility of macular disease. With the expanding base of both functional testing and structural imaging tools, optometrists are better equipped than ever to make the diagnosis and begin treatment of these potentially sight-threatening conditions.
Dr. Reynolds is an associate professor of optometry and Nova Southeastern University College of Optometry. She is currently the instructor for the clinical medicine course and lectures on a variety of topics in ocular disease, She is a member of the Optometric Retina Society.
|
1. Friedman D, O’Colmain B, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States: Arch of Ophthal. 2004;122(4):564–72. 2. National Eye Institute. Eye Disease Factsheet. Available at: https://nei.nih.gov/sites/default/files/neipdfs/NEI_Eye_Disease_Statistics_Factsheet_2014_V10.pdf. Accessed January, 2016 3. Rosen RB, Hathaway M, Rogers J, et al. Multidimensional en-face OCT imaging of the retina. Opt Express. 2009;17:4112-4133. 4. Do D, Gower E, Cassard S, et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC study. Ophthalmology. 2012;119:771–8. 5. Kotsolis A, Killian F, Ladas I, Yannuzzi L. Fluorescein angiography and optical coherence tomography concordance for choroidal neovascularization in multifocal choroiditis. Br J Ophthalmol. 2010;94:1506–8. 6. de Carlo T, Romano A, Waheed N, Duker J. A review of optical coherence tomography angiography (OCTA). International Journal of Retina and Vitreous. 2015;(1)5:1-15. 7. Schmitz-Valckenberg S, Holz F, Bird A, Spaide R. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385-409. 8. Von Rückmann A, Fitzke F, Bird A. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995 May;79(5):407-12. 9. Kellner U, Renner A, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47(8):3531-8. 10. Bian Q, Gao S, Zhou J, et al. Lutein and zeaxanthin supplementation reduces photo-oxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radical Biology & Medicine. 2012;53:1298–307. 11. Bernstein P, Delori F, Richer S, et al. The value of measurement of macular carotenoid pigment optical densities and distribution in age-related macular degeneration and other retinal disorders. Vision Research. 2010;50(7):716-28. 12. Ma L, Yan S, Huang Y, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012;19(11):2290–7. 13. The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–36. 14. The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Ophthamol. 2013;09:2005-15. 15. Everdell N, Styles I, Calcagni A, et al. Multispectral imaging of the ocular fundus using light emitting diode illumination. ReSciInstrum. 2010;81(9):093706. 16. Hood D, Frishman L, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–85. 17. Jurklies B, Weismann M, Husing J. Monitoring retinal function in neovascular maculopathy using multifocal electroretinography: early and long-term correlation with clinical findings. Graefes Arch Clin Exp Ophthalmol. 2002;240:244–64. 18. Lyons J, Severns M. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143:801-9. 19. Maturi R, Yu M, Weleber R. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol. 2004;122:973-81. 20. Jackson G, Scott I, Kim I, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Mar 10;55(3):1427-31. 21. Fujii G, De Juan E, Humayun M, et al. Characteristics of visual loss by scanning laser ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularisation secondary to age related macular degeneration. Am J Ophthamol. 2003;136:1067-78. 22. Colby S, Ortman J. Projections of the size and composition of the US Population: 2014 to 2060 (US Census Bureau, March 2015). P.5. 23. AMD Alliance International. Basic facts about AMD. Available at: http://www.amdalliance.org/information_overview_basic_facts.html. Accessed March 2016 24. Joachim N, Mitchell P, Kifley A, Wang J. Incidence, progression, and associated risk factors of medium drusen in age-related macular degeneration: Findings from the 15-year follow-up of an australian cohort. JAMA Ophthalmol. 2015 Jun;133(6):698-705. 25. Seddon J, Reynolds R, Yu Y, et al. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011;118:2203–11. 26. Sui G, Liu G, Liu G. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97:389–394. 27. Haan M, Klein R, Klein B, et al. Hormone therapy and age-related macular degeneration: the Women’s Health Initiative Sight Exam Study. Arch Ophthalmol. 2006;124(7):988- 92. 28. Klein R, Zeiss C, Chew E. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. 29. Jakobsdottir J, Conley Y, Weeks D, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389–40. 30. Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004 Aug;242(8):690-8. 31. Steel D, Lotery A. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye. 2013;27:1–21. 32. Gass J. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752-9. 33. Duker J, Kaiser P, Binder S. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013 Dec;120(12):2611-9. 34. de Bustros S, Vitrectomy for Prevention of Macular Hole Study Group. Vitrectomy for prevention of macular holes. Results of a randomized multicenter clinical trial. Ophthalmology. 1994;101:1055-9. 35. Hikichi T, Yoshida A, Akiba J, Trempe C. Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. Br J Ophthalmol. 1995;79:517-20. 36. Niwa H, Terasaki H, Ito Y, Miyake Y. Macular hole development in fellow eyes of patients with unilateral macular hole. Am J Ophthalmol. 2005;140:370-5. 37. Michaelides M, Stover N, Francis P, Weleber R. Retinal toxicity associated with hydroxychloroquine and chloroquine. Arch Ophthalmic. 2011 Jan;129(1):30-9. 38. Marmor M, Carr R, Easterbrook M, et al. American Academy of Ophthalmology recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377-82. 39. Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009;93(11):1444-7. 40. Chen E, Brown D, Benz M, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol. 2010;4:1151-8. 41. Mititelu M, Wong B, Brenner M, et al. Progression of hydroxychloroquine toxic effects after drug therapy cessation: New evidence from multimodal imaging. JAMA Ophthalmol. 2013;131:1187-97. 42. Marmor M, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132(9):1105-12. 43. Garweg J, Wenzel A. Diabetic maculopathy and retinopathy. Functional and sociomedical significance. Ophthalmologe. 2010;107:628–35. 44. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: An extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991;98:786-806. 45. Hee M, Puliafito C, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Archives of Ophthalmology. 1995;113(8):1019–29. 46. Helmy Y, Allah H. Optical coherence tomography classification of diabetic cystoid macular edema. Clin Ophthalmol. 2013;7:1731–7. 47. Cheung N, Mitchell P, Wong T. Diabetic retinopathy. Lancet. 2010;376:124–36. 48. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012 Jun;11:1-7. |
