A Practical Approach to Angle-closure
Learn to triage these patients and how to intervene appropriately with in-office treatments or swift referrals as needed.
By Michael Cymbor, OD, and Nicole Stout, OD
 |
Release Date: July 15, 2020
Expiration Date: July 15, 2023
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe clinical factors that define chronic, intermittent and acute angle-closure.
- Explain the differences between primary and secondary angle-closure.
- Discuss how the various classifications affect the long-term outcomes.
- Manage angle-closure patients.
- Determine when to refer patients for surgical management.
Target Audience: This activity is intended for optometrists engaged in the care of patients with angle-closure.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Michael Cymbor, OD, Nittany Eye Associates, and Nicole Stout, OD, Northeastern State University Oklahoma College of Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 68530-GL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Cymbor receives fees for non-CME/CE services from Optovue.
Dr. Stout has no disclosures.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
| Annual Glaucoma Report Check out the other feature articles in this month's report: Glaucoma: The Perils of Progression Seven Ways Glaucoma Care is Changing |
When a patient complains of recent eye injection, deep eye pain and nausea, and has an intraocular pressure (IOP) of 52mm Hg, our first thought is angle-closure crisis, and every optometrist takes an “all hands on deck” approach until the crisis is under control. However, the steps necessary in the more common scenario of the asymptomatic patient presenting with an IOP of 18mm Hg with narrow angles are much less clear.
This article reviews the medical and surgical treatments of angle-closure over the continuum of the disease. Angle-closure is not a single diagnosis but rather a spectrum.1 The urgency and treatment should reflect the location on this spectrum.
Angle-closure by the Numbers
Angle-closure glaucoma (ACG) affects approximately 23 million people, and the number is expected to increase to 32 million by 2040.2 ACG is responsible for nearly half of all blindness caused by glaucoma worldwide.3
When it comes to angle assessment in the management of glaucoma, several older terms such as occludable, sub-acute, latent or intermittent, may not be helpful given there is a lack of consensus on their meaning.
Angle-closure disease may be primary, secondary or, more likely, a combination of both. It may occur suddenly as an angle-closure crisis, or be chronic, progressing slowly over the course of years. Although a traditional approach views angle-closure as a single disease entity, angle-closure is a heterogenous disease involving different mechanisms that should be identified by the clinician.
Case Example
A 63-year-old white male was referred to a glaucoma specialist by his local optometrist due to increasing IOP and worsening glaucoma. The patient’s mother, father and brother all have glaucoma. He reported taking 0.2% brimonidine BID and 0.5% timolol qAM, both OU, for several years.
Best-corrected visual acuity was 20/30 OD and 20/25 OS. He had an afferent pupillary defect OD. His IOP was 23.7mm Hg OD and 21.8mm Hg OS. His corneal hysteresis was 7.3 OD and 7.8 OS. Central corneal thickness was 523µm OD and 530µm OS. Visual field testing revealed a severely reduced field OD>OS with a mean defect of 22.6 OD and 14.8 OS.
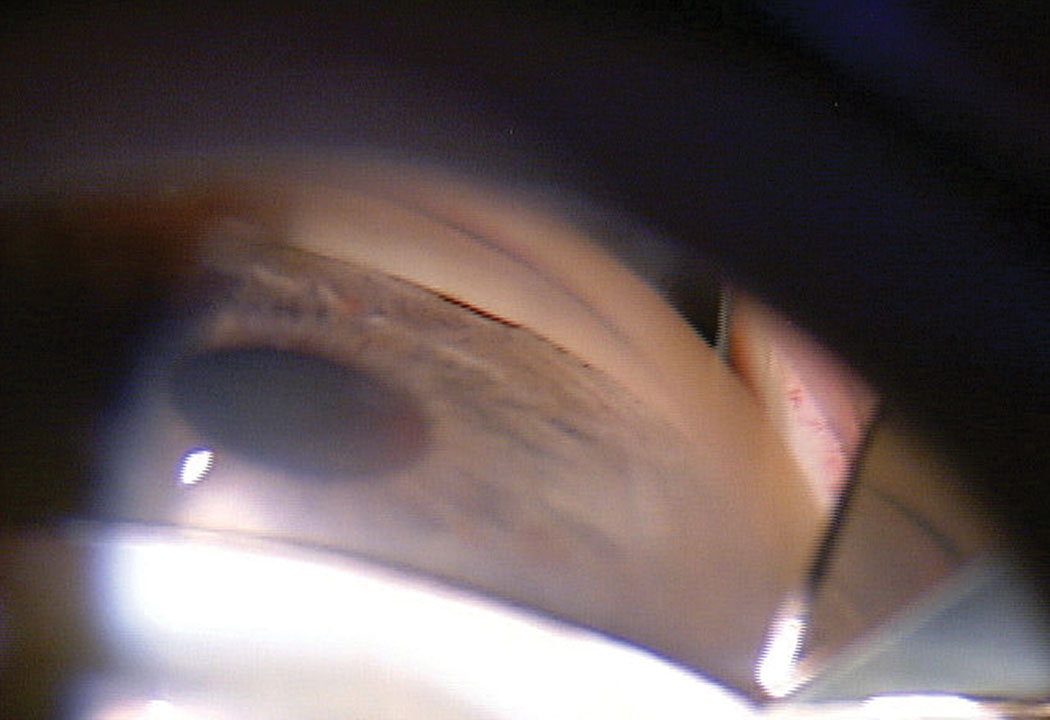 |
| Fig 1. This patient’s initial gonioscopy shows a narrow angle with the TM barely visible. Click image to enlarge. |
Optical coherence tomography (OCT) revealed severely reduced retinal nerve fiber layer and ganglion cell complex OD and moderately reduced OS. OCT angiography showed reduced vessel density OD>OS.
The cup-to-disc ratio was graded at 0.8/0.8 OD and 0.65/0.65 OS. Gonioscopy showed minimal trabecular meshwork (TM) visible with grade 2 pigment OU in all quadrants (Figure 1). Anterior segment OCT (AS-OCT) confirmed narrow angles (Figure 2).
The patient was diagnosed with severe angle-closure glaucoma OD and moderate ACG OS, staged based on the visual field defect. We performed a bilateral YAG laser peripheral iridotomy (LPI). This had little effect on IOPs or angle opening. We proceeded with cataract surgery with the hope of also performing goniosynecialysis and Kahook Dual Blade goniotomy (New World Medical).
Primary vs. Secondary
Primary angle-closure (PAC) covers a broad spectrum of angle disease. The common feature to all primary angle-closure is the presence of narrow drainage angles characterized by the apposition of the TM and the peripheral iris. The currently accepted classification system in primary angle-closure disease is primary angle-closure suspect (PACS), PAC and primary angle-closure glaucoma (PACG).4 PACS includes patients who have greater than 180 degrees of iridotrabecular contact with a normal IOP and no optic nerve damage. PAC has greater than 180 degrees of iridotrabecular contact with peripheral anterior synechiae (PAS) or elevated IOP but no optic neuropathy. PACG has everything contained with PAC along with glaucomatous optic neuropathy or the presence of glaucomatous visual field defects.5
Secondary ACG occurs as a result of an underlying pathological process. It can be classified as resulting from an anterior “pulling” mechanism by which the peripheral iris is pulled into the angle, occluding the TM, such as:1-7
- Neovascular membrane forming in the anterior chamber angle secondary to retinal ischemia, which can occur in conditions such as proliferative diabetic retinopathy, central retinal vein occlusion, central retinal artery occlusion and ocular ischemic syndrome;
- PAS secondary to inflammation that can occur following anterior segment surgery or in chronic uveitis;
- Endothelial membrane obstructing the angle in iridocorneal endothelial (ICE) syndrome or posterior polymorphous corneal dystrophy; or
- Epithelial membrane from epithelial downgrowth following ocular trauma.
Conversely, it may also occur through a posterior “pushing” mechanism where the iris or ciliary body is pushed forward to occlude the angle, such as:1-7
- Absolute pupillary block occurring when 360 degrees of posterior synechiae cause iris bombe (a form of secondary pupillary block). This occurs as a result of inflammatory conditions, such as uveitis, that cause the iris to fibrose to the anterior surface of the lens, impeding the normal flow of aqueous;
- Lens-induced angle-closure through subluxation, anterior lens displacement, malpositioning of an intraocular lens or phacomorphic glaucoma (all forms of secondary pupillary block);
- Aphakic pupillary block, which occurs as a result of anterior vitreous displacement and adhesion between the vitreous humor and the iris (a form of secondary pupillary block);
- Ciliary body cysts or tumors, which can cause anterior displacement of the peripheral iris;
- Posterior segment space-occupying lesions, such as tumors, silicone oil or a gas bubble, that cause anterior displacement of the lens-iris diaphragm;
- Choroidal effusion, which most commonly occurs as a complication following glaucoma surgery, but may also be secondary to other intraocular surgeries, inflammatory or infectious diseases, trauma, neoplasms, drug reactions (topiramate and sulfonamide-induced angle-closure), venous congestion or idiopathic uveal effusion; or
- Ciliary block (also known as aqueous misdirection), which causes shallowing of the anterior chamber as a result of aqueous humor being misdirected into the vitreous body displacing the lens-iris diaphragm forward. This condition can occur following ocular surgery.
Secondary angle-closure can involve an aspect of secondary pupillary block or can occur without pupillary block.6-11
However, most cases of angle-closure are due to pupillary block, which occurs when movement of the aqueous from the posterior to anterior chamber is halted, creating a pressure gradient that leads to forward bowing of the peripheral iris, resulting in sudden obstruction of the TM. Of all acute angle-closure patients in the United States, 90% present with pupillary block.12
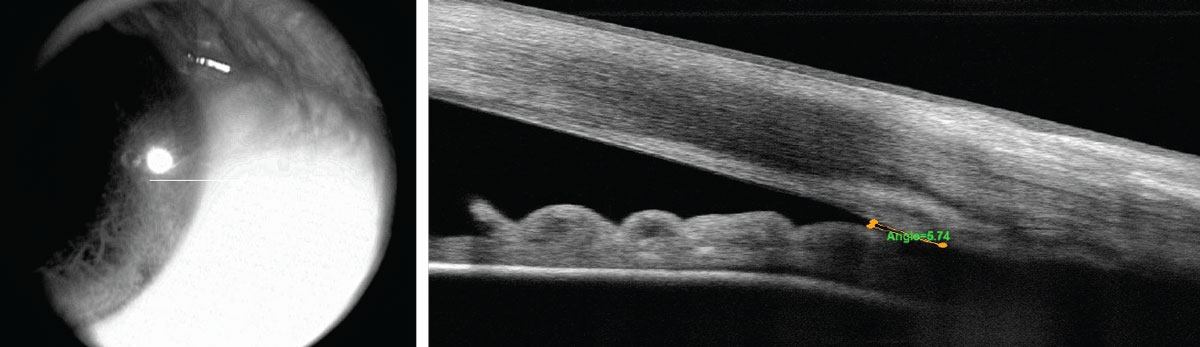 |
| Fig. 2. The patient’s initial OCT shows a narrow angle. Click image to enlarge. |
Diagnosing Angle Issues
Four factors help clinicians diagnose potential angle issues: symptoms, signs, risk factors and angle assessment. A careful history and clinical exam are necessary to make the proper diagnosis.
Symptoms of a primary or secondary angle-closure crisis include eye redness, reduced vision, halos, ocular or periocular pain, nausea and vomiting.3 While symptoms are common in an angle-closure crisis, most cases of chronic angle-closure are asymptomatic.13 Ocular signs include elevated IOP, conjunctival injection with ciliary flush, corneal edema and a mid-dilated pupil.3
Demographic risk factors include advancing age, female gender and Asian ancestry.14,15 Asian populations typically have thicker irises with a more anterior lens position.16 Ocular risk factors include hyperopia, shallow anterior chamber, small anterior chamber volume and area, thicker peripheral iris and a higher insertion, increased lens vault and an anterior ciliary body position.17,18
Gonioscopy is still the standard when evaluating angle structures, and clinicians must be intimately familiar with angle assessment. Unfortunately, angle assessment may be among the most underused aspects of glaucoma management. One study found that 40% of diagnosed open-angle glaucoma patients actually had angle-closure.19 Other studies show that gonioscopy/angle assessment is performed less than 50% of the time in glaucoma patients and suspects.20,21
When the angle is open, the most posterior angle structure visible is the ciliary body (CB), found between the iris root and the scleral spur. It is usually brown but may appear as light gray. The second most posterior structure is the scleral spur and can vary in color from white to gray. It is found in the posterior margin of the scleral sulcus, between the CB and the TM. The scleral spur is comprised of collagen tissue and serves as the anchor for the ciliary muscle.
The TM is next, found between the scleral spur and Schwalbe’s line. It can be subdivided into anterior and posterior TM. It is typically light gray in younger patients and becomes more pigmented in older individuals. The anterior third of the TM is nonfunctional, while the posterior two-thirds filters aqueous into Schlemm’s canal. Schwalbe’s line is the most anterior angle structure and represents the end of a clear cornea. While there are three main angle classification systems—Scheie, Shaffer and Spaeth—the universally accepted classification system is to simply describe the most posterior structure seen by quadrants.22-24
Gonioscopy is critical for identifying some causes of secondary angle-closure. Indentation gonioscopy can help clinicians differentiate between iridocorneal apposition and peripheral anterior synechia. This technique is performed using a small-diameter gonioscopy lens to apply pressure to the central cornea, displacing the aqueous humor towards the angle, which separates the iris from the cornea and allows for better visualization of the angle structures. Angle structure visibility with indentation suggests iridocorneal apposition, while synechial angle-closure should not improve angle structure visibility upon indentation.
While gonioscopy remains the standard, technologies such as AS-OCT, ultrasound biomicroscopy (UBM) and Scheimpflug imaging are playing a more prominent role as more doctors gain access. Furthermore, angle-closure diagnosis rates increase when objective analysis is included.25 AS-OCT acquires a high-resolution cross-sectional image of the anterior chamber. It often shows the angle narrower than gonioscopy, particularly in the superior and inferior quadrants.25 This may be due to OCT’s ability to measure angles in scotopic conditions. One disadvantage of AS-OCT is that current devices only sample a small section of the angle at one time.
UBM is also an excellent tool for imaging the anterior segment and can be helpful in identifying the underlying pathology; however, it is not readily available in most private practices.6,7,26 UBM has the advantage of being able to image behind the iris, including the lens and the CB, but is costly because it is typically a stand-alone instrument.
Scheimpflug imaging may also play a role in angle imaging. Scheimpflug imaging can sample a much larger portion of the angle, but the resolution is less than either AS-OCT or UBM.27
While each technology has advantages, objective angle analysis complements gonioscopy.
Acute Treatment Approaches
Treatment of acute angle-closure crisis is typically prompt medical stabilization followed by laser and/or surgical stabilization.
Medical stabilization. This may include treatment with topical alpha agonists, beta blockers, carbonic anhydrase inhibitors and rho-kinase (ROCK) inhibitors. Medical treatment may also include topical steroids to relieve inflammation. Oral treatment may include carbonic anhydrase inhibitors. This approach should be avoided in topiramate- or sulfonamide-induced angle-closure; instead, the causative medication should be discontinued promptly.7,10
Oral or intravenous hyperosmotic medications may be used when rapid IOP lowering is not achieved with the above-mentioned treatments. Compression gonioscopy performed with a small-diameter lens may be necessary to break recent iridotrabecular adhesion. In absolute pupillary block, clinicians should use a strong cycloplegic agent and 10% phenylephrine ophthalmic solution to try and break the posterior synechia, in addition to pharmacological interventions that attempt to lower IOP and control inflammation.
 |
| Fig. 3. This image depicts the patient’s angle after goniosynechialysis, cataract surgery and Kahook Dual Blade goniotomy. Click image to enlarge. |
In practical terms, optometric medical stabilization means achieving a significant in-office IOP decrease until an LPI can be performed the same day. Medical stabilization should be tailored to how quickly the LPI can be performed. Once an angle-closure crisis is identified, the optometrist should immediately investigate LPI options and have a clear idea of when it can be performed. If the LPI can be performed immediately on-site or at a referral destination close by, medical stabilization might mean putting in a round of pressure-lowering drops and/or a dose of acetazolamide prior to the LPI. If it cannot be performed until a few hours later, clinicians should put more emphasis on medical stabilization as to not subject the patient to a prolonged elevated IOP.
Laser treatment. Once medical stabilization is achieved and the iris can be visualized, the next step would historically be LPI. If the optometrist practices in a state that permits optometric LPI, the optometrist would then perform an emergent LPI, which is generally effective at relieving pupillary block.
If LPI does not open the angle and decrease IOP, plateau iris syndrome (PIS) should be suspected. PIS is when a large or anteriorly positioned CB pushes the peripheral iris forward, potentially closing the angle. This may be present in up to one-third of angle-closure cases.28 Plateau iris can occur with or without pupillary block. Compression gonioscopy is critical in the diagnosis and will show a marked peripheral iris roll. This occurs because the iris follows the anatomy of the lens from central to peripheral and rises after the level of the equatorial lens up to the anteriorly placed or enlarged CB. UBM may be helpful to visualize the anteriorly positioned ciliary processes.
Argon laser peripheral iridoplasty may help open the angle.29 This technique applies laser to the peripheral iris, reducing its thickness and pulling it away from the TM. Laser iridoplasty may also reverse recent PAS.30 Prompt lens extraction surgery may also be considered. Single-pass four-throw pupilloplasty, which reconstructs the pupil, can be an option in persistent cases.31
Most secondary angle-closure glaucomas with a pupillary block component will require an LPI. Approximately 25% of patients with pupillary block will continue to show iridotrabecular contact even after LPI.32 Factors that may adversely affect LPI success include eyes with greater than 180 degrees of PAS, higher baseline IOP and narrower angles as determined by UBM and AS-OCT.33 Even if LPI is initially successful, it should not be viewed as a long-term cure. The natural lens will continue to grow, narrowing the anterior chamber over time and increasing lens vault.34
Once LPI stabilization of both the angle and IOP occurs, the clinician faces several options. The patient may only require observation with the initiation of topical medication or adjustment of current therapy upon IOP increase.
Provided that the TM is visible to at least 180 degrees, selective laser trabeculoplasty (SLT) may help stabilize IOP. SLT may be limited in angles with 180 degrees or more of PAS and if the TM experiences significant IOP-induced trauma during acute PAC. There appears to be no difference in SLT outcomes between patients with PAC and PACG.35 Transscleral (delivery through the pars plana) and endoscopic cyclophotocoagulation are also options, as they reduce CB aqueous formation and shrink the CB.36
Surgical treatment. Even though LPI is the most historically common choice of treatment after medical stabilization, moving directly to lens extraction may be the better option.
The EAGLE study compared LPI with clear lens extraction by looking at patients over 50 years old with mild to moderate PACG with a presenting IOP above 30mm Hg.37 The study found a reduction in the need for further medications or glaucoma surgeries in the clear lens extraction group along with a better quality of life and better cost-effectiveness. A recent study suggests that lens extraction should be performed early as a way to prevent PACG.4 In the case of phacomorphic glaucoma, cataract surgery should be performed as the definitive treatment.10
Phacoemulsification with intraocular lens implantation may relieve iridotrabecular contact, lowering IOP. In some cases, goniosynecialysis may be needed to break contact. This involves mechanically disrupting PAS by gently pushing on the peripheral iris to break the attachment between the iris and the TM.
If successful, a variety of TM-targeting minimally invasive glaucoma surgeries (MIGS) may then be employed, such as Kahook Dual Blade goniotomy, Trabectome (Microsurgical Technolo) and iStent (Glaukos). Trabeculectomies and tubes are also an option for more advanced cases. As with our patient, Kahook Dual Blade and goniosynecialysis combined with phacoemulsification can provide reductions in both IOP and the need for IOP-lowering medications.38
If phacoemulsification, and possibly goniosynecialysis, does not relieve iridotrabecular contact, the optometrist may need to refer the patient for a more aggressive MIGS such as the Xen gel stent (Allergan). A trabeculectomy or a tube procedure may also be needed but both have more postoperative complications for angle-closure than primary open-angle glaucoma patients.39
Secondary angle-closure considerations. Causes of secondary ACG due to posterior “pushing” mechanisms that do not involve pupillary block are often a result of the peripheral iris being displaced forward by the lens or CB. In these cases, the use of a cycloplegic agent to induce posterior rotation of the CB is often indicated, in addition to topical IOP-lowering drops and topical steroids.7
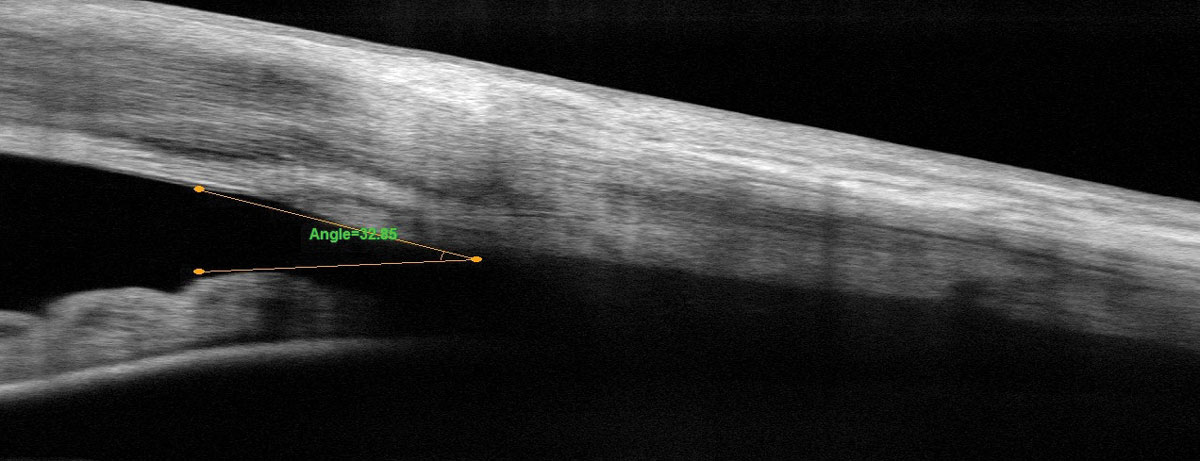 |
| Fig. 4. This is the patient’s corresponding AS-OCT after successful treatment. Click image to enlarge. |
Many of these conditions require a referral to a glaucoma, retina or ocular oncology specialist to manage the underlying cause. Although pilocarpine can be used in primary phakic pupillary block glaucoma to pull the peripheral iris away from the TM, it can cause contraction of the ciliary muscle, resulting in anterior lens movement and paradoxical worsening of the angle-closure in cases of secondary ACG.40
Secondary angle-closure glaucoma resulting from anterior “pulling” mechanisms also often requires a referral for surgical intervention, such as in the case of secondary ACG caused by significant PAS where goniosynecialysis could be performed. This procedure is more likely to be successful if the synechia are relatively new.7,10
In neovascular glaucoma, after attempting to get the IOP and inflammation under control with pharmacological therapies, the patient should be referred to a retina specialist for treatment of the underlying retinal ischemia with panretinal photocoagulation and/or anti-vascular endothelial growth factor agents. These patients will often also require a referral to a glaucoma specialist for more invasive glaucoma surgeries such as a tube procedure.7,8,10
After the acutely elevated IOP is lowered and the underlying cause of the primary or secondary ACG has been treated, clinicians should monitor these patients regularly with IOP checks, optic nerve head assessments, OCTs, angle assessments and visual fields to monitor for further glaucomatous progression and to detect if additional intervention becomes necessary.
Caring for the Chronic Patient
While an acute angle-closure crisis is a clinical emergency requiring immediate care, chronic angle-closure may be more insidious and progress slowly. It remains a clinical challenge to determine the ideal time to intervene. For instance, questions persist regarding whether LPI should be recommended for all PACS patients to prevent PAC and/or PACG.
The recent ZAP trial showed a statistically significant but clinically small decrease in the risk of PAC conversion and recommended against the widespread use of prophylactic LPIs in their study population.41 Further analysis of the ZAP trial found that 44 PACS patients needed treatment to prevent one new PAC case over six years.42
LPI is mostly benign, usually opens the angle to some extent and potentially prevents an angle-closure crisis. Nevertheless, side effects may occur, including dysphotopsia and accelerated cataract formation.43,44
In our clinic, we typically follow most asymptomatic PACS patients every six to 12 months. We monitor for changes in the angle, optic nerve and visual field. While we approach each patient individually, we generally perform LPI if the patient mentions symptoms suggestive of closure, has a family history of angle-closure or if they show progression of angle narrowing.
Eyes that develop PAC or PACG should be treated.33 The treatment of chronic angle-closure is similar to the treatment for acute angle-closure: stabilize the IOP medically or with SLT, evaluate the angle, perform LPI when appropriate and consider cataract or clear lens extraction (PACG cases) with or without MIGS. The clinician should escalate therapy when progression is identified. Chronic angle-closure treatment may follow a course of years, rather than days or months. Patients with PAC or PACG who are followed closely and treated more aggressively than primary open-angle glaucoma patients generally have favorable long-term outcomes.45
Our Patient
Fortunately, phacoemulsification combined with goniosynecialysis opened our patient’s angle enough to proceed with Kahook Dual Blade goniotomy (Figure 3). These three procedures stabilized aqueous outflow and IOP. One year later, his IOP is 11.4mm Hg OD and 10.4mm Hg OS on no medications. His fields and optic nerve OCTs are stable along with his AS-OCTs (Figure 4).
As optometrists continue to play a more significant role in all aspects of glaucoma management, it is critical that we better appreciate the importance of angle assessment, use all of our angle diagnostic options, refer patients when appropriate and monitor and manage these patients over the course of their lives.
Dr. Cymbor is the medical director of the Glaucoma Institute of State College, a member of the Optometric Glaucoma Society and a managing partner at Nittany Eye Associates.
Dr. Stout is an assistant professor at Northeastern State University Oklahoma College of Optometry.
1. Cumba RJ, Nagi KS, Bell NP, et al. Clinical outcomes of peripheral iridotomy in patients with the spectrum of chronic primary angle closure. ISRN ophthalmology. 2013;2013:828972. 2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7. 3. Weinreb RN, Friedman DS, eds. Angle Closure and Angle Closure Glaucoma - Consensus Series Book 3. Amsterdam: Kugler Publications; 2006:1-61. 4. Song MK, Sung KR, Shin JW, et al. Glaucomatous progression after lens extraction in primary angle closure disease spectrum. J Glaucoma. May 1, 2020. [Epub ahead of print]. 5. Prum BE, Herndon LW, Moroi SE, et al. Primary angle closure preferred practice Pattern guidelines. Ophthalmology. 2016;123(1):P1-40. 6. Weizer JS. Angle-closure glaucoma. UpToDate. Waltham, MA, 2020. 7. Kremer FZ, Chadha N, Tai TY, et al. Secondary angle closure: imaging, diagnosis, etiology, and treatment. Advances in Ophthalmology and Optometry. 2017;2(1):301-19. 8. Parivadhini A, Lingam V. Management of secondary angle closure glaucoma. J Curr Glaucoma Pract. 2014;8(1):25-32. 9. American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel: Primary Angle Closure PPP – 2015. www.aao.org/preferred-practice-pattern/primary-angle-closure-ppp-2015. Accessed June 22, 2020. 10. Gerstenblith AT, Rabinowitz MP, eds. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. 11. Teekhasaenee C, Dorairaj S, Ritch R. Secondary angle-closure glaucoma. In: Shaaraway TM, Sherwood MB, Hitchings RA, Crowston JG, eds. Glaucoma. 2nd Edition. Elsevier Inc. 2015;401-9. 12. Ritch R, Lowe RF, Reyes A. Angle-closure glaucoma: therapeutic overview. The Glaucomas. 1996;2:1521-31. 13. Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238-42. 14. Cheng JW, Zong Y, Zeng YY, et al. The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PLoS One. 2014;9(7):e103222. 15. Bonomi L, Marchini G, Marrafa M, et al. Epidemiology of angle-closure glaucoma. Prevalence, clinical types, and association with peripheral anterior chamber depth in the Egna-Neumarkt Glaucoma Study. Ophthalmol. 2000;107(5):998-1003. 16. Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118(3):474-9. 17. Moghimi S, Fathollahzadeh N, Chen R, et al. Comparison of fellow eyes of acute primary angle closure and phacomorphic angle closure. J Glaucoma. 2019;28(3):194-200. 18. Mansouri M, Ramezani F, Moghimi S, et al. Anterior segment optical coherence tomography parameters in phacomorphic angle closure and mature cataracts. Invest Ophthalmol Vis Sci. 2014;55:7403-9. 19. Vijaya L, George R, Baskaran M, et al. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population: the Chennai Glaucoma Study. Ophthalmology. 2008;115(4):648-54. 20. Fremont AM, Lee PP, Mangione CM, et al. Patterns of care for open-angle glaucoma in managed care. Arch Ophthalmol. 2003;121(6):777-83. 21. Stanley J, Huisingh CE, Swain TA, et al. Compliance with primary open-angle glaucoma and primary open-angle glaucoma suspect preferred practice patterns in a retail-based eye clinic. J Glaucoma. 2018;27(12):1068-72. 22. Scheie HG. Width and pigmentation of the angle of the anterior chamber: a system of grading by gonioscopy. AMA Arch Ophthalmol. 1957;58(4):510-2. 23. Becker B, Shaffer RN. Diagnosis and Therapy of the Glaucomas. St. Louis: CV Mosby; 1965:42-53. 24. Spaeth GL. The normal development of the human anterior chamber angle: a new system of descriptive grading. Trans Ophthalmol Soc UK. 1970;91:709-39. 25. Sakata LM, Lavanya R, Friedman DS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115(5):769-74. 26. Maslin JS, Barkana Y, Dorairaj SK. Anterior segment imaging in glaucoma: an updated review. Indian J Ophthalmol. 2015;63(8):630-40. 27. Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: a new era for ophthalmic diagnosis? Br J Ophthalmol. 2007;91(4):551-7. 28. Kumar RS, Baskaran M, Chew PT, et al. Prevalence of plateau iris in primary angle closure suspects: an ultrasound biomicroscopy study. Ophthalmol. 2008;115(3):430-4. 29. Leong JC, O’Connor J, Ang GS, et al. Anterior segment optical coherence tomography changes to the anterior chamber angle in the short-term following laser peripheral iridoplasty. J Curr Glaucoma Pract. 2014;8(1):1-6. 30. Sun X, Liang YB, Wang NL, et al. Laser peripheral iridotomy with and without iridoplasty for primary angle-closure glaucoma: 1-year results of a randomized pilot study. American J Ophthalmol. 2010;150(1):68-73. 31. Narang P, Agarwal A, Kumar DA. Single-pass four-throw pupilloplasty for angle-closure glaucoma. Indian J Ophthalmol. 2018;66(1):120-4. 32. Jiang Y, Chang DS, Zhu H, et al. Longitudinal changes of angle configuration in primary angle-closure suspects: the Zhongshan Angle-Closure Prevention Trial. Ophthalmology. 2014;121(9):1699-705. 33. Radhakrishnan S, Chen PP, Junk AK, et al. Laser peripheral iridotomy in primary angle closure: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(7):1110-20. 34. Lee KS, Sung KR, Shon K, et al. Longitudinal changes in anterior segment parameters after laser peripheral iridotomy assessed by anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3166-70. 35. Raj S, Tigari B, Faisal TT, et al. Efficacy of selective laser trabeculoplasty in primary angle closure disease. Eye. 2018;32(11):1710-6. 36. Liu GJ, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd: YAG laser cyclophotocoagulation. Ophthalmic Res. 1994;26(2):65-79. 37. Javanbakht M, Azuara-Blanco A, Burr JM, et al. Early lens extraction with intraocular lens implantation for the treatment of primary angle closure glaucoma: an economic evaluation based on data from the EAGLE trial. BMJ Open. 2017;7(1):e013254. 38. Dorairaj S, Tam MD. Kahook dual blade excisional goniotomy and goniosynechialysis combined with phacoemulsification for angle-closure glaucoma: 6-month results. J Glaucoma. 2019;28(7):643-6. 39. Sihota R, Gupta V, Agarwal HC. Long‐term evaluation of trabeculectomy in primary open angle glaucoma and chronic primary angle closure glaucoma in an Asian population. Clin Exp Ophthalmol. 2004;32(1):23-8. 40. Ritch R. The pilocarpine paradox. J Glaucoma. 1996;5(4):225-7. 41. He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. The Lancet. 2019;393(10181):1609-18. 42. Gupta V, Dada T. Should we perform peripheral laser iridotomy in primary angle closure suspects: implications of the ZAP trial? Ann Transl Med. 2019;7(Suppl 3):S157. 43. Spaeth GL, Idowu O, Seligsohn A, et al. The effects of iridotomy size and position on symptoms following laser peripheral iridotomy. J Glaucoma. 2005;14(5):364-7. 44. Vijaya L, Asokan R, Panday M, et al. Is prophylactic laser peripheral iridotomy for primary angle closure suspects a risk factor for cataract progression? The Chennai Eye Disease Incidence Study. Br J Ophthalmol. 2017;101(5):665-70. 45. Sihota R, Sood A, Gupta V, et al. A prospective longterm study of primary chronic angle closure glaucoma. Acta Ophthalmol Scand. 2004;82(2):209-13. |
