Thyroid Disease: A Delicate Balance Disrupted
This diagnosis isn’t as cut-and-dry as you think; be prepared to manage both hyper- and hypothyroid patients.
By
Release Date: February 2018
Expiration Date: January 17, 2021
Goal Statement: Thyroid dysfunction can manifest in many ways—all of which can negatively impact patients. A deeper understanding of the thyroid gland and its many dysfunctions, particularly hypothyroidism, is essential for maintaining patients’ ocular health and quality of life. This article will help ODs better understand the various thyroid conditions and their ocular effects.
Faculty/Editorial Board: Matt Dixon, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 56365-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Iannucci and Francesca Crozier-Fitzgerald all have no relationships to disclose.
The thyroid gland is critically important for our overall health and function—for one, hormones produced by the thyroid gland play a major role in energy production and metabolism at the cellular level. Every tissue and organ requires optimal thyroid hormone (TH) levels to work properly, especially the heart and brain.1
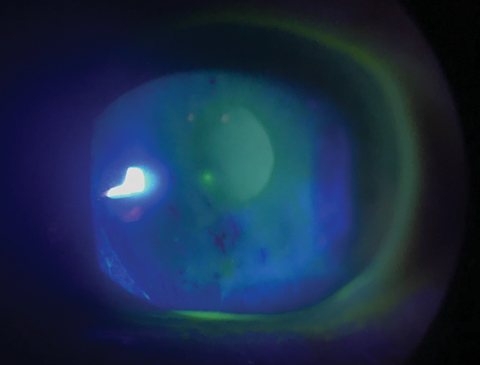 |
| Dry eye is the most common ocular side effect of both hyper- and hypothyroidism. Click image to enlarge. |
While optometrists are prepared to recognize Graves’ disease, a major cause of hyperthyroidism, thyroid dysfunction can manifest in other ways as well—all of which can negatively impact patients. A deeper understanding of the thyroid gland and its many dysfunctions is essential for not only a more holistic view of our patients, but also improved ocular health and quality of life.
The American Thyroid Association (ATA) suggests thyroid disorders affect at least 20 million Americans, and 60% of patients with the disorder are unaware.2 Moreover, more than 12% of Americans will develop a thyroid condition during their lifetime.2 These numbers emphasize the inevitability of one of these patients seeking care in your office. Even once patients are diagnosed, their symptoms often linger. One study found that, among patients taking thyroid medication, only 60% were within the normal hormone range.3 These researchers concluded that “thyroid dysfunction is common, may often go undetected and may be associated with adverse health outcomes that can be avoided by serum thyroid stimulating hormone (TSH) measurement.”3
This article will help you better understand both hyper- and hypothyroidism and the ocular effects, as well as how to detect, treat and monitor these patients.
The Ins and Outs of the Thyroid
An elaborate feedback loop involving the hypothalamus and pituitary glands controls the output of the thyroid, making up the hypothalamic–pituitary–thyroid (HPT) axis. When the body requires energy to perform a specific function, requiring TH, the hypothalamus releases thyrotropin-releasing hormone (TRH), which initiates the production of TSH in the pituitary gland and signals the production of thyroxine (T4). Serum T4 is monitored by the HPT axis, which, in normal individuals, will generate the correct amount of TH.
 |
| Image: The Diabetes Council |
T4 is known as a “storage hormone” because it is inactive and not a direct source of energy. T4 is converted into triiodothyronine (T3) as the necessary source of energy for the body.1 The normal thyroid gland will generate 90% T4 and only about 10% T3. Most of the conversion to T3 occurs in other tissues in the body such as the brain, kidney and liver.1
Nutrients such as iodine and selenium play a key role in this conversion. The body requires about 60mcg of iodine daily for adequate production of TH, and the average intake in the United States is about 1,000mcg.4 However, about a third of the world’s population live in iodine-deficient areas and are at an increased risk of iodine deficiency disorders such as goiter, hypothyroidism, mental disability, increased perinatal mortality and retarded physical development.5 Selenium is also crucial in thyroid health, as it acts as one of the catalysts for the conversion of T4 to T3 and then helps to control thyroid hormone metabolism.6
Hyper vs. Hypo
The thyroid can malfunction in two ways: by creating too much TH, hyperthyroidism, or by creating too little, hypothyroidism (HT). Each of these conditions can cause significant systemic and ocular effects, and it’s critical to know which one is at play when caring for these patients.
| In-Office Numbers |
Hyperthyroidism. Between 1% and 3% of Americans are diagnosed with hyperthyroidism, with Graves’ disease as the number one cause in the United States, where iodine is sufficient.7 The second leading cause is toxic multinodular goiter, in which the nodules become a source of TH independently and do not respond to TSH signaling.7
Graves’ disease is characterized by the presence of hyperthyroidism, goiter and, sometimes, ophthalmopathy and dermopathy. The cause of Graves’ disease is thought to be multifactorial, but researchers believe it arises, in part, from the loss of immunotolerance and the development of auto-antibodies that stimulate thyroid follicular cells by binding to the TSH receptor.8 These antibodies upregulate the receptor by mimicking TSH, causing excessive TH production.
Symptoms of hyperthyroidism include heart palpitations, fatigue, tremors, anxiety, disturbed sleep, weight loss, heat intolerance, sweating and polydipsia.
Hyperthyroidism can be confirmed by low serum TSH, elevated T4 and T3 or both. Ultrasound and radioactive iodine uptake may also aid in diagnosis.
Hypothyroidism. Clinical experience suggests underactive thyroid is the more common of the two thyroid dysfunctions; however, data regarding prevalence is lacking and varies greatly between countries. A landmark study based in the United Kingdom found a prevalence of around 9.5%.9 The Colorado Thyroid Disease Prevalence Study also found that 9.5% of individuals had a TSH above 5.1mIU/L, while also acknowledging the diagnostic challenges of establishing accurate numbers in the United States.3
Research does show that women are five times more likely than men to have HT, and women with HT are twice as likely to have a heart attack than women without the disease. In addition, 6% of miscarriages are associated with HT, and autism and low IQ in pediatric patients are linked to HT during pregnancy.10,11
Hashimoto’s thyroiditis is the most common cause of HT, followed by thyroidectomy, amiodarone-induced hypothyroidism and postpartum thyroiditis. As much as 95% of patients with HT have Hashimoto’s disease.12
| A Complete Thyroid Function Test | ||
| Test | Significance | Normal Adult Ranges* |
| TSH | This is released by the pituitary gland and controls the production of thyroid hormone. | 0.5mIU/mL to 3.0mIU/mL (differs in pregnancy) |
| Total T4 | This is a pro-hormone, which is converted into T3 by 5’-iodinase (rarely measured). | 5μg/dL to 10.8μg/dL |
| Total T3 | Checks levels of triiodothyronine, the active thyroid hormone (rarely measured). | 75ng/dL to 200ng/dL |
| Free T4 | Only a small fraction of the circulating hormone is free (unbound) and biologically available, hence measuring concentrations of free thyroid hormones is of greater diagnostic value. | 0.7ng/dL to 2.5ng/dL |
| Free T3 | The most active thyroid hormone. | 2.5pg/mL to 6.5pg/mL |
| Reverse T3 | This is the biologically inactive form of T3. Conversion from T3 to rT3 can be protective in periods of illness or to protect from elevated T3 in hyperthyroidism. In hypothyroidism, high rT3 indicates problems such as too much T4 and not enough T3. | 9ng/dL to 35ng/dL (Genova Diagnostics) |
| Thyroglobulin | The storage form of TH, this is primarily used as a tumor marker in thyroid cancer. | Athyrotic: <0.1ng/mL Intact thyroid: ≤33ng/mL (Mayo Clinic) |
| TPO and TGAb | Thyroid peroxidase antibody (anti-TPO) is the most common test for autoimmune thyroid disease; it can be detected in Graves’ disease or Hashimoto’s thyroiditis. Thyroglobulin antibody (anti-TGAb) targets thyroglobulin. | TPO: 0.0IU/mL to 150IU/mL TGAb: <4.0IU/mL |
| TRAb, TSHRAb, TSI | Thyroid receptor antibody, TSH receptor antibody and thyroid stimulating immunoglobulin are helpful in suspected Graves’ disease. | |
| *Observed dried blood spot ranges based on collected laboratory data. Adapted from ZRT Laboratory. www.zrtlab.com/images/documents/Essential%20Thyroid%20Sample%20Report%202015.pdf. Accessed December 15, 2017 | ||
In most cases of Hashimoto’s thyroiditis, blood tests will reveal one or two types of anti-thyroid antibodies: thyroid peroxidase antibody (in up to 95% of those with Hashimoto’s) and antibodies against thyroglobulin (around 80%). Because these antibodies may appear decades before a change in TSH, screening is always crucial in suspected thyroid disease.12
| Masquerading Disease Graves’ Disease Patients have TSH receptor antibodies (TRab). Tiredthyroid.com. Accessed November 30, 2017. |
Symptoms of HT are numerous and include feeling cold, depression, fatigue, anxiety, hair loss, dry skin and eyes, thinning eyebrows temporally, brain fog and impaired memory, high cholesterol, slow pulse, irregular menstrual cycles, insomnia, edema and constipation. Practitioners can uncover most of these concerns with a symptom questionnaire (from the Institute for Functional Medicine, for example) for every confirmed or suspected HT patient.13 Anecdotally, most patients taking medication for HT will confirm as many as 10 residual symptoms that can be linked to underactive TH, and they frequently list dry, gritty eyes as one of the symptoms.
This disease is also known to exacerbate adverse effects of heart disease, hypertension, elevated cholesterol, cognitive deficits, autism, infertility, fibroids and neuromuscular dysfunction. One study found that, as thyroid function decreased, LDL cholesterol went up. Of patients taking thyroid medication, 40% still had abnormal TSH values—suggesting a need for better monitoring and disease control.3
Despite these findings, the American Academy of Family Physicians has concluded that routine HT screenings are not helpful.14
The ATA and the American Association of Clinical Endocrinology (AACE) consider TSH to be the best marker for diagnosing and treating HT. However, no consensus exists on normal TSH reference ranges. More than a decade ago, the National Academy of Clinical Biochemistry recommended an upper limit of 2.5mIU/L because 95% of normal individuals fall under this value.15 They recognized compounding factors such as age, gender and pregnancy, as well as a diurnal variation of as much as 50%, with levels being highest during sleep.15
However, the 2014 ATA guidelines define subclinical (mild) HT as elevated TSH greater than 10mIU/L and a normal free T4, while overt (severe) HT is characterized by an elevated TSH greater than 10mIU/L and abnormal free T4.16 Although it makes sense that hypothyroidism is acquired slowly over time, treatment is reserved until TSH reaches a level the individual clinician considers abnormal. For patients with signs and symptoms of HT, close monitoring of labs (including antibodies that indicate autoimmune involvement) may help doctors detect this disease before serious consequences occur.
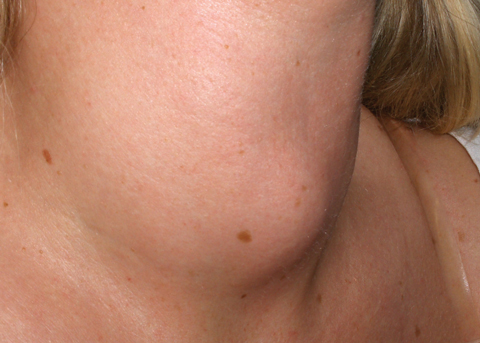 |
| Patients with Graves’ disease may present with hyperthyroidism, goiter (seen here) and, sometimes, ophthalmopathy and dermopathy. Image: Drahreg01, wikimedia commons |
While some doctors may not treat the disease until TSH is above 10mIU/L, this upper limit has been falling in recent years, and many labs now set it at 4.5mIU/L. Regardless of the number, making treatment decisions based only on the preferred lab’s normative range for TSH, ignoring the entire clinical picture and especially symptoms, often leads to underdiagnosis.
ODs should inquire about the patient’s last TSH level, as they would for HbA1c in diabetes patients, but remember it should be viewed on an individual basis in light of symptoms and other factors.
Ocular Involvement
Depending on whether a patient has too much or too little TH in their body, they can present with any number of ocular side effects, often beginning with dry eyes.
Hyperthyroidism. Graves’ disease, an autoimmune state, may lead to thyroid-associated orbitopathy (TAO), formerly known as thyroid eye disease, in 25% of patients. Approximately 80% of TAO cases are associated with hyperthyroidism, although the onset may not coincide with the onset of the hyperthyroid state. Euthyroid TAO refers to the 5% to 10% of Graves’ disease patients who do not develop hyperthyroidism. The final 10% of TAO patients have primary autoimmune hypothyroidism.17
Typically, the first sign of TAO is ocular surface disease (OSD), although patients may also have eyelid retraction/proptosis, lagophthalmos, periorbital edema and diplopia. TAO may appear prior to diagnosis of a thyroid condition, so ordering a thyroid panel and comanaging with an endocrinologist is necessary. Still, orbital involvement progresses independently of thyroid disease and at various phases. In most cases, TAO is self-limiting.
The most serious risk of TAO is optic neuropathy with vision loss. While optometrists should seek comanagement with a specialist, they should also provide supportive measures to reduce edema, limit photophobia and protect the ocular surface. Management also includes CT or MRI to rule out optic neuropathy from compression related to the enlargement of the extraocular muscles. In addition, visual field testing may reveal defects caused by optic neuropathy.
| Case Example She was referred back to her endocrinologist with a recommended change in therapy to natural desiccated thyroid or similar. She was switched to Armour Thyroid (levothyroxine, liothyronine, Allergan) 60mg once daily. Many of her symptoms improved within a few months. After two years she no longer uses Restasis, and her OSDI score has improved to 14. |
To date, no effective means of preventing Graves’ disease or reliably altering its course exist. Current therapeutic options for mild TAO include observation, patient education, lifestyle changes (i.e., smoking cessation, salt restriction, sun protection) and ocular surface lubrication. Symptoms of moderate disease can be managed with topical cyclosporine, nighttime eyelid taping, moisture goggles, prism glasses or selective ocular patching and moderate-dose oral steroid therapy.17,18
Active ocular involvement often requires the use of systemic corticosteroids to manage inflammation. Depending on side effects and patient tolerance, they may be tapered over three to six months.19
Once a patient has progressed to severe disease, high-dose corticosteroids, external beam radiation and steroid-sparing immunosuppressive agents for reducing the inflammation may be necessary. In addition, surgery may be indicated to correct the residual abnormalities secondary to fibrosis in the inactive state of the disease. These interventions are aimed at the consequences of the disease, rather than targeting its cause. Unfortunately, they do not prevent or reverse the pathological changes in the orbital tissues.17,18
Hypothyroidism. Optometrists likely see a high percentage of HT patients due to signs and symptoms of significant dry eye disease (DED), which is extremely common in this patient population. While studies documenting the association between OSD and Graves’ orbitopathy are numerous, those linking HT and DED are lacking. However, one study that identified DED comorbidities revealed that patients with DED were twice as likely to have HT compared with patients without DED.20
Systemic Treatment
Patients diagnosed with a thyroid disorder can pursue any number of therapies ranging from medical management to surgical intervention:
Hyperthyroidism. While surgical thyroidectomy is the most successful treatment for Graves’ disease, medicinal options include the antithyroid drugs propylthiouracil and Tapazole (methimazole, Pfizer). Beta-blockers may also be needed in cases of thyroid storm, or thyrotoxic crisis, which is an acute, life-threatening, hypermetabolic state induced by excessive release of TH in individuals with thyrotoxicosis. The clinical features are fever, tachycardia, hypertension and neurological and GI abnormalities.21
Another possible treatment is radioactive iodine therapy, although it is contraindicated for moderate to severe Graves’ orbitopathy.
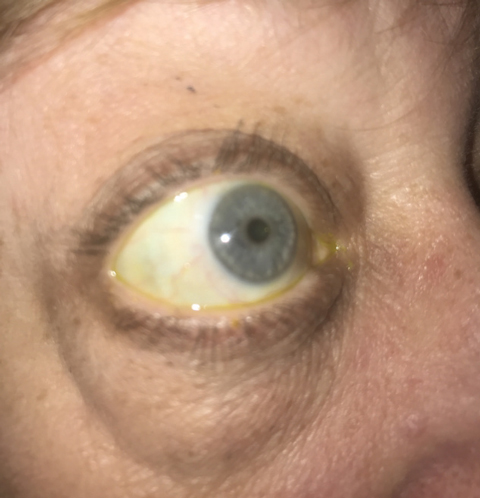 |
| Graves’ ophthalmopathy often presents with exopthalmos, or bulging eyes, as seen in this patient. |
Hypothyroidism. Patients with Hashimoto’s disease can manage many of their symptoms nutritionally by addressing selenium and vitamin D deficiencies and by maintaining proper iron and iodine levels. Patients should also consider eliminating gluten from their diet, as research suggests gluten intolerance, or Celiac’s disease, is associated with Hashimoto’s thyroiditis.22
Oxidative stress is also a factor in Hashimoto’s, and efforts to increase antioxidant status could be helpful.23 Specifically, glutathione, perhaps the body’s most potent endogenous antioxidant, is reduced in Hashimoto’s. While direct supplementation of glutathione is often ineffective, n-acetylcysteine (NAC) supplementation can aid in the endogenous biosynthesis of glutathione. NAC and alpha lipoic acid are excellent strategies to lower oxidative stress.24 Nuclear factor-erythroid 2 p45-related factor-2 (Nrf2) activation, a mechanism for cellular defense, also increases antioxidant production, such as glutathione, in response to reactive oxygen species.25
Although HT as a whole is really a deficiency of active T3 in tissues such as the eye, patients are treated based on the TSH output from the pituitary gland, in response to TRH from the hypothalamus. However, evidence suggests a normal TSH may still exist when serum T3 is low, indicating only specific tissues are in a hypothyroid state.26
 |
| This screening questionnaire is a useful tool for patients at risk for hypothyroidism or those currently taking thyroid medications who have continuing symptoms. Click here for a PDF version. |
HT treatment goals include maintaining “normal” TSH levels and resolution of symptoms. Some guidelines establish a therapeutic goal between 0.4mIU/L and 4.0mIU/L.16 Typically, clinicians seek to regulate TSH and fail to inquire about a patient’s quality of life—perhaps the main reason for continued symptoms such as dry eye, which is difficult to treat with unbalanced TH levels.
Medical management is dominated by monotherapy with levothyroxine (LT4) for all HT conditions, as it is the only recommended treatment by the 2014 ATA and AACE guidelines. LT4 is now among the most prescribed drugs in the United States, ranking in the top two monthly among all prescriptions.27 Few clinicians, including endocrinologists, will go outside ATA guidelines, even when patients fail to reach a euthyroid state. Unfortunately, the therapy often falls short due to the shortcomings of solely relying on TSH to manage the disease.
One study asserts that normalizing T3 should be a “biological priority” and that many patients fail to achieve a normal T3 on LT4 monotherapy, despite a normal TSH.28 When given the option, many patients do well with combination therapy such as Nature-Throid (RLC Laboratories) or Armour Thyroid (levothyroxine, liothyronine, Allergan).
More than 50 years ago, prior to the synthesis of LT4, the only thyroid hormone replacement available was natural desiccated thyroid sourced from porcine thyroid glands. Several brands are still available in the United States today, as is a synthetic T3, available as Cytomel (liothyronine sodium, Pfizer) and Triostat (liothyronine, JHP Pharmaceuticals). Clinicians rarely encounter patients on these products or natural desiccated thyroid. With such discrepancies in the guidelines, clinicians should treat each patient individually and take into account not only TSH levels, but also patient symptoms and quality of life.29
Optometrists are certainly familiar with a Graves’ disease diagnosis and are comfortable in the role we play in its treatment and monitoring. However, patients with subclinical or uncontrolled hypothyroidism are far more common in optometric practice than you might think. We can have a life-long impact on thousands of patients if we learn to recognize the implications of the various thyroid dysfunctions and help patients better understand their treatment options. Take a functional approach and take an interest in getting to the root cause, especially when it comes to the top symptom, DED.
| Clinical Pearls However, when patients say their blood work is fine, they might mean TSH is in the “normal” range—practically a moving target. TSH normative ranges vary and must be in the optimal range for each patient, just as we select a target IOP for our glaucoma patients. TSH is the best marker we have, but not the only biochemical parameter that clinicians should monitor. Other biochemical markers, such as free T3, can provide more pertinent information. When multiple symptoms or clinical signs of DED exist, patients on thyroid medication who report “normal” TH levels may not be on optimal treatment that ensures sufficient T3. These uncontrolled patients are common in our practices and appreciate a conversation about the possible relationship to their thyroid condition. Clinicians should open a line of communication with the treating provider to confirm whether or not the patient is compliant with treatment. This is a great time to inquire about recent laboratory testing, as it will aid in the decision making process for concomitant ocular therapies. Here is a sample letter to a treating clinician you can use in your office: MD, NP, PA: I am treating our mutual patient ______________ for chronic dry eye disease. Studies have shown a correlation with hypothyroidism and dry eye. As we consider treatment options for this patient, would you mind sharing their thyroid lab results? Please include whether or not the TSH target has been obtained, and if Hashimoto’s disease is present based on antibody levels. I appreciate the opportunity to share in the management of this patient. You may fax lab results to xxx-xxx-xxxx. Sincerely, Your comanaging optometrist |
1. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355-82. |
