 |
Evaluation and Diagnosis of Pupil Disorders
Follow this hands-on approach and learn from an expert
clinician to improve your own practice.
By Denise Goodwin, OD
Release Date: February 15, 2023
Expiration Date: February 15, 2026
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
Recognize the pathophysiology of pupil abnormalities.
Create a work-up for the abnormal pupil patient.
Identify when additional tests and/or monitoring are required.
Manage patients with pupil abnormalities.
Target Audience: This activity is intended for optometrists engaged in pupil disorder management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Denise Goodwin, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #125379 and course ID 82783-NO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Author: Dr. Goodwin has no relevant financial interests to disclose. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
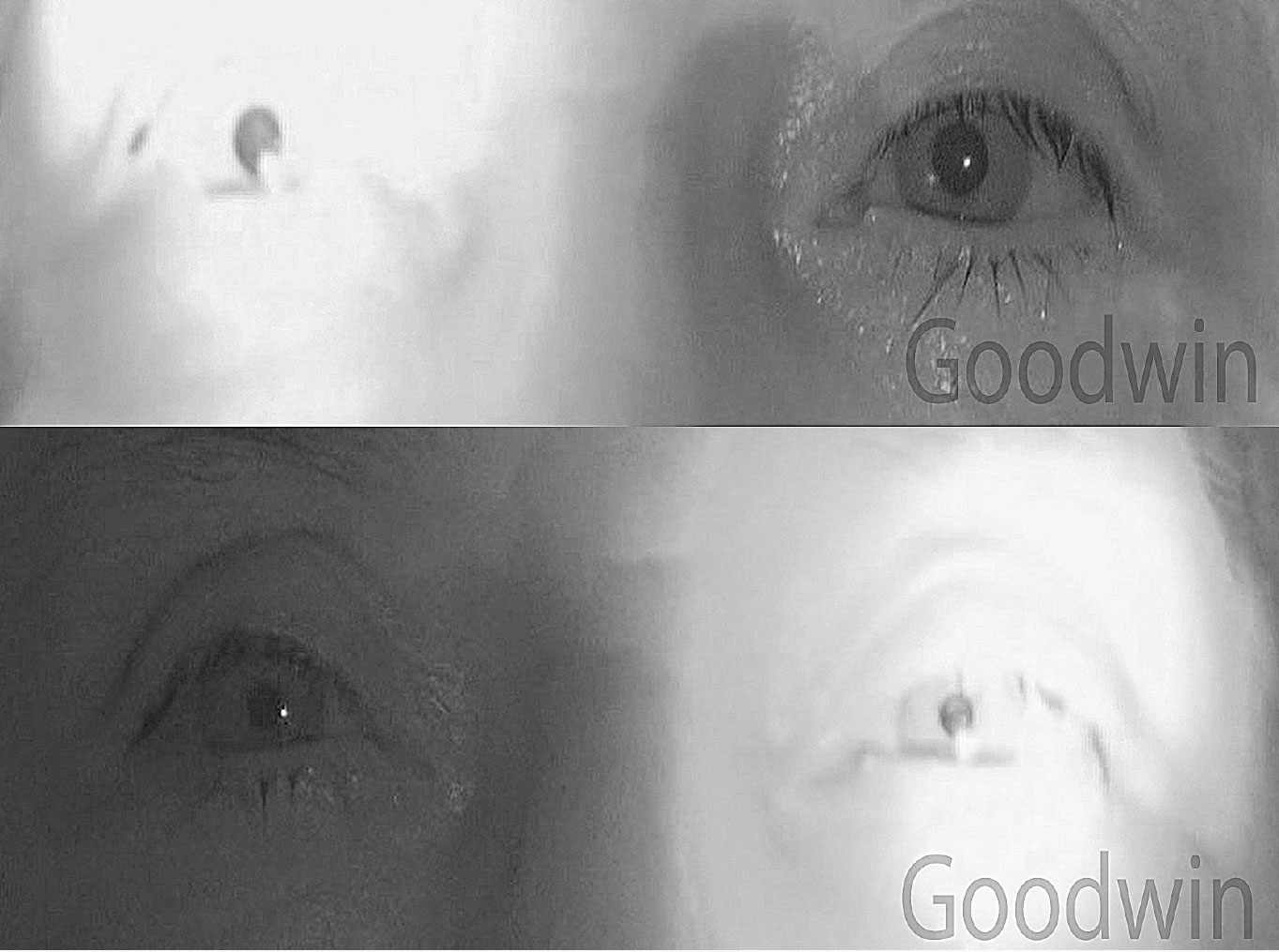 |
Fig. 1. A right RAPD in a patient with optic neuritis. Shining light in the left eye causes both pupils to constrict. Shining light in the right eye causes both pupils to dilate.8 Click image to enlarge. |
Understanding pupil testing and disorders is critical. With one quick, objective test, the pupil evaluation provides information about the retina, optic nerve, chiasm, optic tract, midbrain, cranial nerves III and V and the orbit. In addition, almost all of the true emergent conditions we deal with in optometry can be picked up by a pupil examination.
The following article will cover how to evaluate the pupils and ways to identify and localize both afferent and efferent pupillary disorders. Most importantly, we will cover the characteristics of five pupil disorders that should be managed emergently.
Pupil Evaluation
Check pupil sizes and reactions with the room lights off in order to make the room as dark as possible. The contrast of the lack of room light compared with the brightness of the transilluminator will accentuate a relative afferent pupillary defect. The patient should fixate on a distant target to prevent the pupil constriction that occurs with accommodation and convergence. Look for anisocoria in dim illumination by holding the light two to three inches below the chin and shining the transilluminator upward, tangential to the patient’s face. This will allow you to see the pupils in dim illumination without getting too much light getting in the way. Then use the transilluminator to check pupillary light reactions. While doing this, look for anisocoria in bright illumination.
Finally, check for a relative afferent pupillary defect (RAPD) by moving the light back and forth between both eyes (swinging flashlight test). While alternating the light, count to three. Watch to see at which number the pupils start to dilate. If one pupil starts to dilate at two and the other pupil starts to dilate at three, this is an RAPD. Watching how quickly the pupils redilate during the swinging flashlight test helps to differentiate a subtle RAPD from hippus, which should be symmetric movement between the two eyes.
 |
| Fig. 2. A neutral density bar can be used to determine the severity of an RAPD. Click image to enlarge. |
RAPDs
This finding indicates that the signal going back to the midbrain is unequal between the two eyes. There is no anisocoria associated with an RAPD because both pupils dilate equally when light is shined in the abnormal eye. Shining light in the relatively normal eye causes both pupils to constrict. When counting to three for each eye during the swinging flashlight test, the relatively normal pupil may stay constricted for the entire count. If you have a fairly severe RAPD, both pupils will dilate almost immediately when moving the light into the relatively abnormal pupil (Figure 1). With a more subtle RAPD, the relatively abnormal pupil will stay constricted and then start to dilate between counts one and two compared with the relatively normal pupil which may stay constricted for the full count of three.
RAPDs can be graded on a scale of zero to four. A grade one RAPD indicates initial constriction followed by quicker dilation of the involved pupil, while a grade two RAPD has no initial movement followed by a quick dilation. Grade three is an immediate dilation of the pupil as you shine light from the normal to the involved eye. With a grade four RAPD, the pupil will stay dilated even with prolonged illumination.
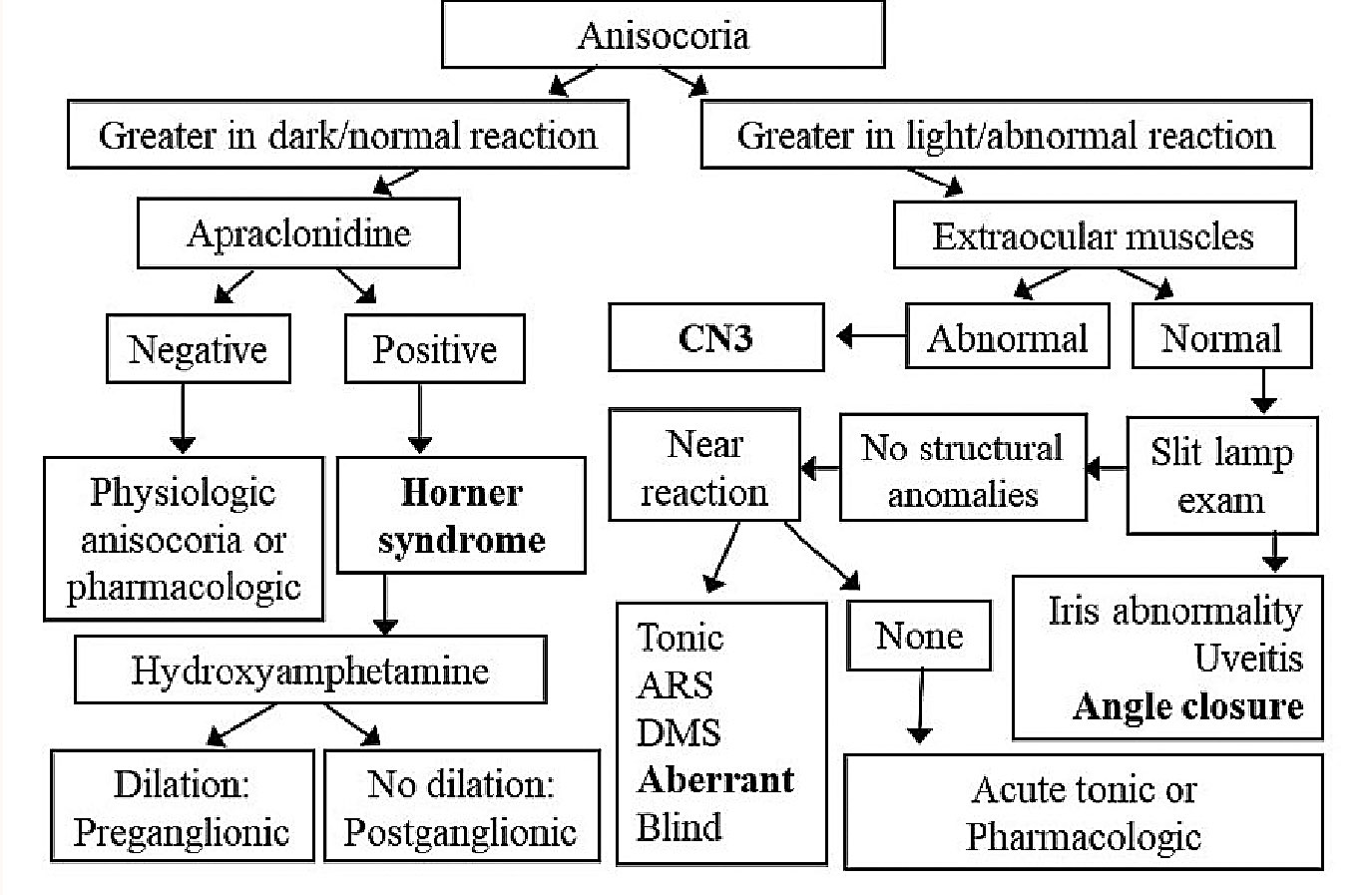 |
Fig. 3. Flowchart differentiating various causes of anisocoria. The bolded conditions indicate situations that need to be dealt with emergently. CN3: cranial nerve III, ARS: Argyll Robertson syndrome, DMS: dorsal midbrain syndrome Click image to enlarge. |
Neutral density filters can be used to quantify an RAPD (Figure 2). Put the filter over the better eye while performing the swinging flashlight test. Continue increasing the density until there is no difference in pupillary dilation.
If one pupil is fixed due to neurologic disease, iris trauma or medication, check for an RAPD using the reverse Marcus Gunn test. Watch only the mobile pupil while alternating the light between the two eyes. If the mobile pupil constricts when light is shined directly in the eye and dilates when light is shined in the opposite eye, there is an RAPD in the nonmobile pupil. If the afferent signal is equal between the two eyes, the mobile pupil constricts when the light is shined directly in the eye and then stays constricted when light is shined in the opposite eye. Thus, there will be no movement of the mobile pupil as you move the light back and forth.
A brachium lesion of the superior colliculus can cause an RAPD with normal vision. Fibers leave the optic tract before getting to the lateral geniculate nucleus and travel to the pretectal nuclei. As these fibers do not go to the lateral geniculate nucleus, vision will be unaffected. It is otherwise fairly unusual that the visual system would be normal when an RAPD is present. Compressive optic neuropathy can cause normal vision and an RAPD because visual acuity loss can be very mild with compressive optic neuropathy despite a significant RAPD. Also, a previous episode of demyelinating optic neuritis can cause normal visual acuity and an RAPD since the acuity goes back to normal after about six weeks, but there is generally permanent loss of ganglion cell fibers.The most common cause of an RAPD is unilateral or asymmetric optic nerve disease. You can also get an RAPD with a large retinal lesion. If there is subtle retinal disease, it will not cause an RAPD. Amblyopia and marked anisocoria can cause a very mild RAPD.1,2 A lesion of the optic tract will cause an RAPD in the eye with the worse visual field. An optic tract lesion with a complete homonymous hemianopia will cause an RAPD in the eye contralateral to the lesion (i.e., the eye with the temporal visual field).3 This occurs in part because there are more intrinsically photosensitive retinal ganglion cells in the nasal vs. the temporal retina.
It is important to know what does not cause an RAPD so it isn’t attributed to an incorrect etiology, causing one to miss a potentially serious condition. An RAPD will not occur with a media opacity, such as corneal scarring or cataract. In fact, an RAPD often occurs in the eye with less severe cataract.4 Subtle retinal lesions will not cause an RAPD. If the lesion is bilateral and symmetric or behind the lateral geniculate nucleus, you will not get an RAPD. No RAPD will be present despite even severe functional vision loss.
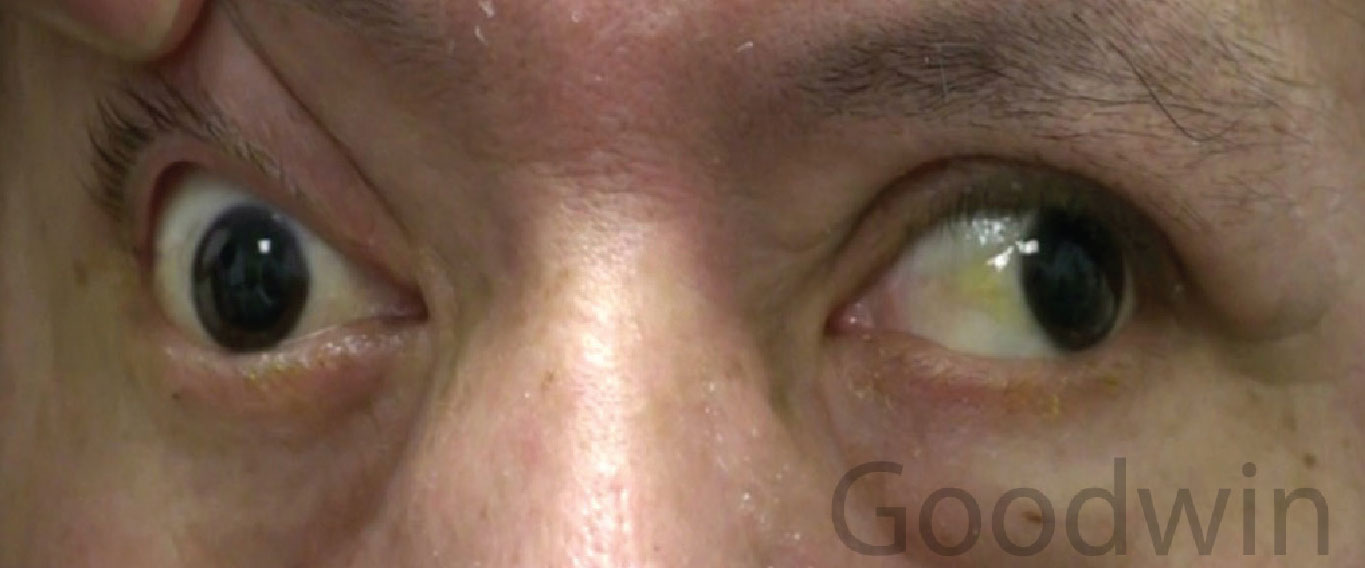 |
| Fig. 4. A right cranial nerve III palsy. The right eyelid is manually raised due to ptosis. The eye is unable to adduct or move up or down. Click image to enlarge. |
Efferent Pupillary Defects
The characteristic feature of an efferent defect, regardless of whether it is a sympathetic or parasympathetic issue, is anisocoria. Figure 3 shows a flowchart that can help determine a diagnosis once anisocoria is detected. Note the four diagnoses that are bolded and need to be dealt with emergently.
If anisocoria is present, observe the pupils in dim and bright illumination. If the anisocoria is worse in bright light, consider a parasympathetic problem. This is verified by looking at the pupil’s reaction to light. A parasympathetic problem causes poor constriction to light. That lack of constriction is what makes the anisocoria worse in bright light. If the anisocoria is worse in dim illumination, an issue with the sympathetic system is most likely present. With a sympathetic problem, the pupil will constrict normally to light, but it will dilate slowly.
Cranial nerve III palsy. If anisocoria is worse in bright light with abnormal reaction to light, look for associated features like ptosis or extraocular muscle palsies. If the muscles are involved, consider a cranial nerve III palsy (Figure 4). This is an emergent situation. A cranial nerve III palsy that involves the pupil should be considered due to an aneurysm until proven otherwise. This presents with a dilated pupil, ptosis, an inability to adduct the eye and an inability to move the eye up or down.
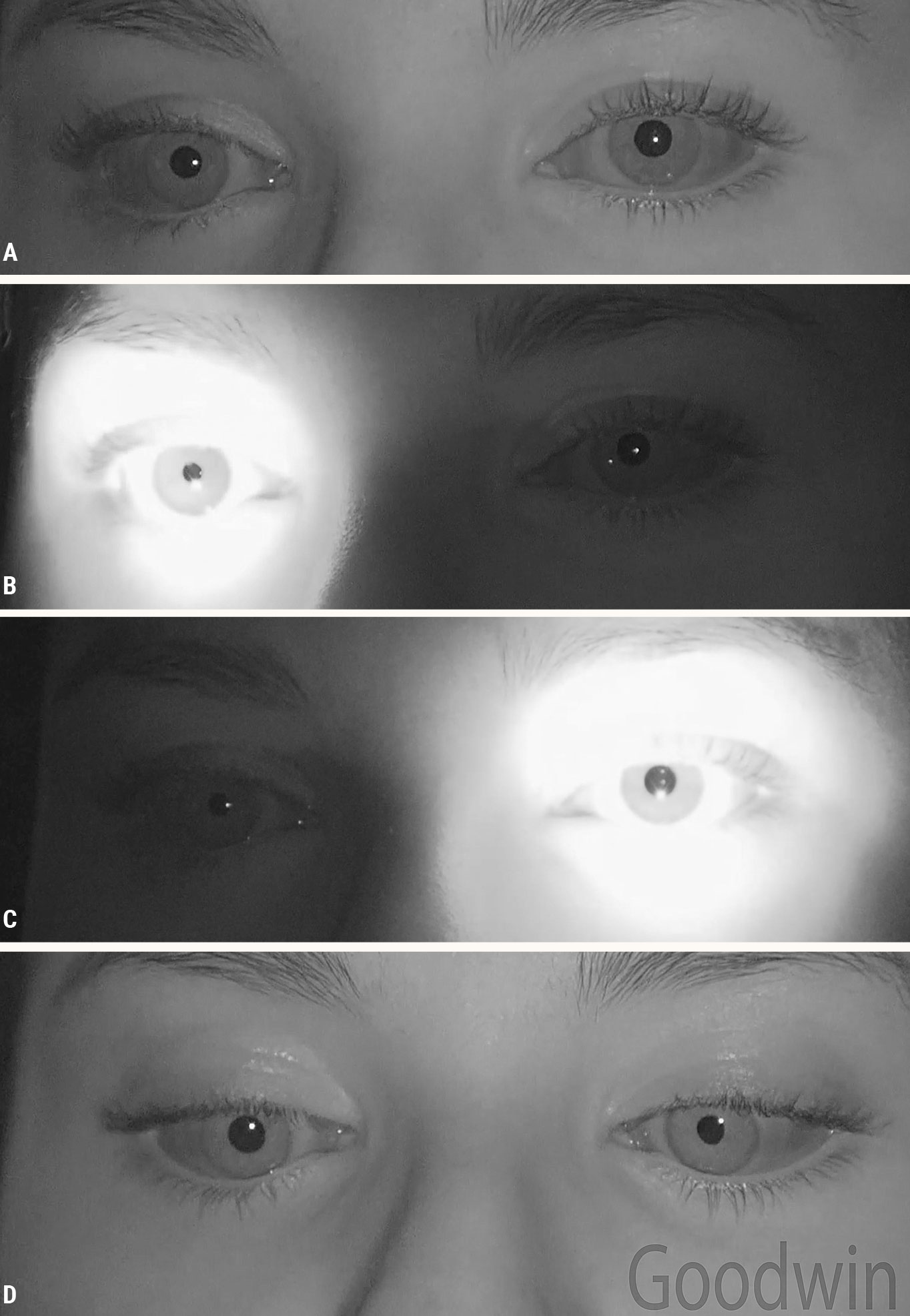 |
| Fig. 5. A left tonic pupil: (A) There is slight anisocoria in dim illumination. (B) The normal right pupil is responsive to light. (C) The tonic left pupil is not responsive to light. When light is shined in the eye the amount of anisocoria is greater. (D) Both eyes constrict to a near target, although the left eye takes longer to constrict maximally. Click image to enlarge. |
Whether or not the pupil is involved indicates the likelihood that an aneurysm is the cause of the cranial nerve III palsy. A complete pupil-sparing cranial nerve III palsy (the pupil is normal) is most likely due to ischemia, as would occur with diabetes. It is possible that the pupil may not be involved yet but may become involve imminently. Don’t dilate a patient with a cranial nerve III palsy, and watch the pupils carefully. If the pupils become involved, immediate computed tomography angiography or magnetic resonance angiography (MRA) is warranted. A pupil-sparing cranial nerve III palsy due to ischemia will generally improve with time and care of the vasculopathic risk factors.
If the pupil is involved in a cranial nerve III palsy, emergent magnetic resonance imaging (MRI) and MRA should be ordered looking for an aneurysm.5 Pupillary fibers are located on the superficial surface of cranial nerve III as it exits the midbrain. The extraocular muscle fibers are deeper in the nerve. Because of this, an aneurysm that is pressing hard enough to cause an extraocular muscle deficit will also compress the pupillary fibers. An incomplete cranial nerve III palsy—for example, only the medial rectus and inferior rectus are involved—requires an MRI, regardless of pupil involvement.
Iris abnormalities. If extraocular muscle movements are normal, look for iris abnormalities. Iris defects may confound the pupillary evaluation. Additionally, there are important iris conditions that should not be missed. Congenital aniridia can be an indication of Wilms tumor—a cancer of the kidney. A coloboma or an ectopic pupil can complicate your pupil exam. Other conditions that can result in pupillary abnormalities include uveitis, iris tumors, trauma and ischemia, which occurs with ocular ischemic syndrome.
Ischemia of the iris occurs with angle-closure glaucoma. This is one of the five emergent causes of pupil abnormalities because of the potential to cause blindness quickly. This will produce a mid-dilated pupil that is not responsive to light or a near target. These patients often have corneal edema, pain, redness and halos around lights. Ultimately, this diagnosis is made by taking intraocular pressure.
Light near dissociation. Any time a patient’s light reflex is not normal, check near pupillary responses. Light near dissociation, where the pupil reacts better to a near target than to light, indicates one of the following five conditions: a tonic pupil, an Argyll Robertson pupil, dorsal midbrain syndrome, aberrant regeneration or a blind eye.
Blindness due to lesions anterior to the lateral geniculate nucleus will cause a lack of light reflex. Even if the patient is completely blind bilaterally, focusing on their own thumb will produce the pupillary constriction that comes with convergence and accommodation.
Aberrant regeneration may be congenital or due to a past trauma. It can also be due to a slow-growing mass, the most emergent of which is an aneurysm. With aberrant regeneration, damaged fibers grow to innervate structures they were not initially intended to innervate. If fibers from the medial rectus aberrantly grow to innervate the sphincter, the pupil will constrict when the patient converges to look at a near target despite not constricting to light due to direct damage. If you know that there was a previous injury or that the aberrant regeneration is congenital, no additional testing is necessary. If the patient has no previous history, the aberrant regeneration indicates a slow-growing mass. Consider an aneurysm until proven otherwise.
 |
| Click table to enlarge. |
Another cause of light near dissociation is a lesion pushing on the posterior midbrain. A tumor of the pineal gland, which sits directly behind the midbrain, can damage the pupillary fibers that travel in the posterior midbrain. Typically, both eyes will become non-reactive to light because the signal does not get from the pretectal nuclei to the Edinger Westphal area. The fibers involved in the near pupillary response don’t leave through the brachium of the superior colliculus; instead, they continue to the lateral geniculate nucleus, the occipital lobe and the frontal eye fields. The information goes directly from the frontal eye fields to the Edinger Westphal area without going through the pretectal nuclei. Because of this, a lesion affecting the dorsal midbrain will spare the near fibers, and both pupils will respond briskly to a near target despite having no light reaction. Other signs of dorsal midbrain syndrome include a superior gaze palsy and convergence retraction nystagmus.
Argyll Robertson syndrome, commonly associated with neurosyphilis, results in very small pinpoint pupils. Like dorsal midbrain syndrome, the pupils do not react to light but respond briskly to a near target. One difference between the two conditions is that dorsal midbrain syndrome has normal-sized pupils.
 |
| Fig. 6. A right tonic pupil. The pupil is not circular. Click image to enlarge. |
The last of the five conditions causing light near dissociation is tonic pupil. This is a parasympathetic post-ganglionic problem, so the lesion is in the ciliary ganglion or short ciliary nerves.5 The pupil fibers have left the rest of cranial nerve III, so there are no other signs of a cranial nerve III palsy, such as ophthalmoplegia or ptosis. Tonic pupil exhibits greater anisocoria in bright illumination (Figure 5). Despite having no constriction to light, there is a slow constriction to a near target. That slow tonic reaction differentiates this from other causes of light near dissociation where the near pupillary reaction is brisk.
Another characteristic feature of tonic pupil is an irregular pupil border (Figure 6). Here, parts of the iris sphincter still function while other parts do not. A related feature is iris streaming, causing pupil constriction only in some areas. When light is shined in the eye, it looks like the pupil border bunches up in one area like a drawstring of a purse. These are the healthy segments of sphincter tightening up and pulling on the non-functioning areas.
A tonic pupil diagnosis is generally based on the clinical features alone, including the slow light near dissociation, irregular pupil border and segmental constriction; therefore, the use of dilute pilocarpine is usually not necessary. Dilute pilocarpine can help distinguish tonic pupil from a pharmacologically dilated pupil. This is made by combining one drop of 1% pilocarpine with eight drops saline to create a 0.12% concentration. A normal pupil will not constrict to 0.12% pilocarpine, but a tonic pupil will (Figure 7).
 |
| Fig. 7. A right tonic pupil: (A) Before 0.12% pilocarpine is instilled. (B) After 0.12% pilocarpine is instilled in both eyes. Note that the right pupil constricts following drop installation whereas the left does not. Click image to enlarge. |
Often, patients with a tonic pupil come in due to either glare or reading problems (since it initially affects accommodation). After a few weeks, accommodation is at least partially restored. Generally, no other workup or treatment is needed for these patients. Plus lenses can be prescribed if accommodative problems are bothersome or persistent. Tinted glasses or low-dose pilocarpine can be helpful if the patient has light sensitivity due to the dilated pupil.
A number of both local and systemic causes can affect the ciliary ganglia or short ciliary nerves. The ganglion is located within the orbit so any orbital infection, inflammation, mass or trauma can cause a tonic pupil. Very commonly, tonic pupil will occur following ocular surgery, most notably damage to the ciliary ganglion during retinal detachment surgery or damage to the short ciliary nerves during retinal photocoagulation. Systemic diseases that affect the autonomic nervous system, such as diabetes, can cause a bilateral tonic pupil. Tonic pupil is commonly idiopathic, called Adie tonic pupil. Like other idiopathic diseases, all causes of tonic pupil should be ruled out prior to diagnosing Adie tonic pupil.
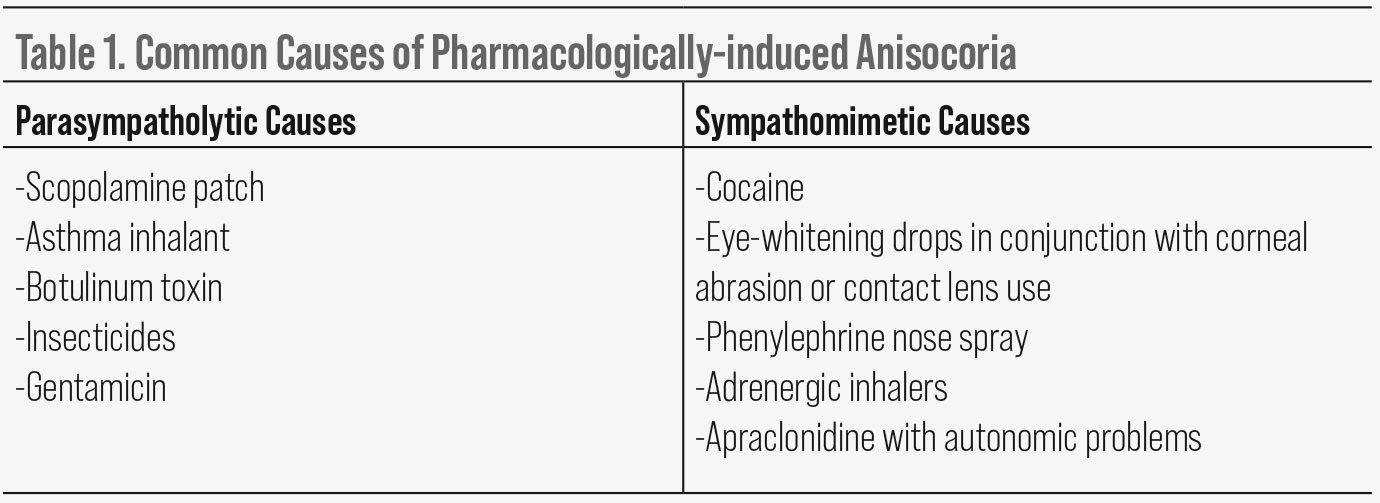
Pharmacologically dilated pupil. If there is poor light reaction, extraocular muscle movements are normal, slit lamp exam is normal and there is no near reaction, consider a pharmacologically dilated pupil or an acute tonic pupil that hasn’t had time to develop aberrant regeneration or hypersensitivity. If due to a parasympatholytic agent, a pharmacologically dilated pupil will not react to light or accommodation. There will be no ptosis or extraocular muscle restriction.
To confirm a pupil is pharmacologically dilated with a parasympatholytic agent, instill a drop of 1% to 2% pilocarpine in the eye. A normal pupil, as well as a tonic pupil or pupil involving cranial nerve III palsy, will constrict with 1% to 2% pilocarpine. A parasympatholytic ally pharmacologically dilated pupil will not constrict. Common causes of pharmacologically dilated pupils are listed in Table 1.
 |
| Fig. 8. A pharmacologically dilated pupil following the use of a sympathomimetic agent. Click image to enlarge. |
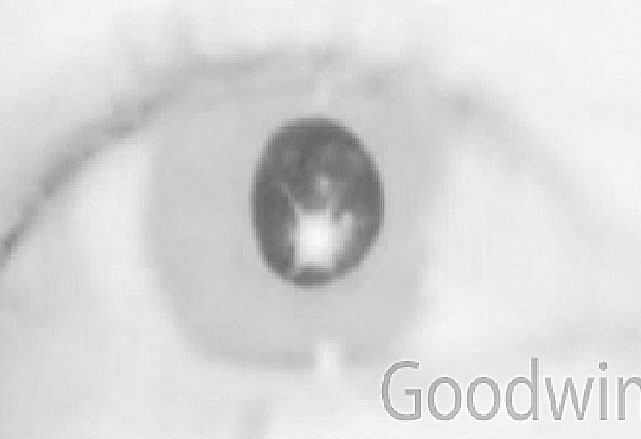
Figure 8 shows a patient with blurry vision in the right eye. He was 20/20 in each eye, but right eye vision wasn’t quite as good as it used to be or as good as the left eye. A careful ocular health exam was normal other than the right pupil being larger than the left. He was using Opcon-A six times per day. A worse depth of focus in the right eye due to the enlarged pupil was considered. He quit using the Opcon-A, and the anisocoria and blurriness went away. This pattern of anisocoria is common in patients using sympathomimetic drops while wearing contact lenses.
Physiologic anisocoria. Figure 9 shows a patient experiencing a particularly severe headache. Her coworker noticed she had different pupil sizes. The anisocoria is slightly more evident in dim illumination, she does not have ptosis, the pupils are reactive to light and the pupils dilate equally. This is consistent with physiologic anisocoria.
Approximately 20% of the population has physiologic anisocoria.5 There are no serious clinical implications. This can be more evident in dim illumination because of mechanical restrictions reducing the anisocoria as the pupils constrict in bright light.6 The mechanical restriction of the smaller pupil gives the larger pupil a chance to catch up. If unsure whether the anisocoria is physiologic, look for a dilation lag.
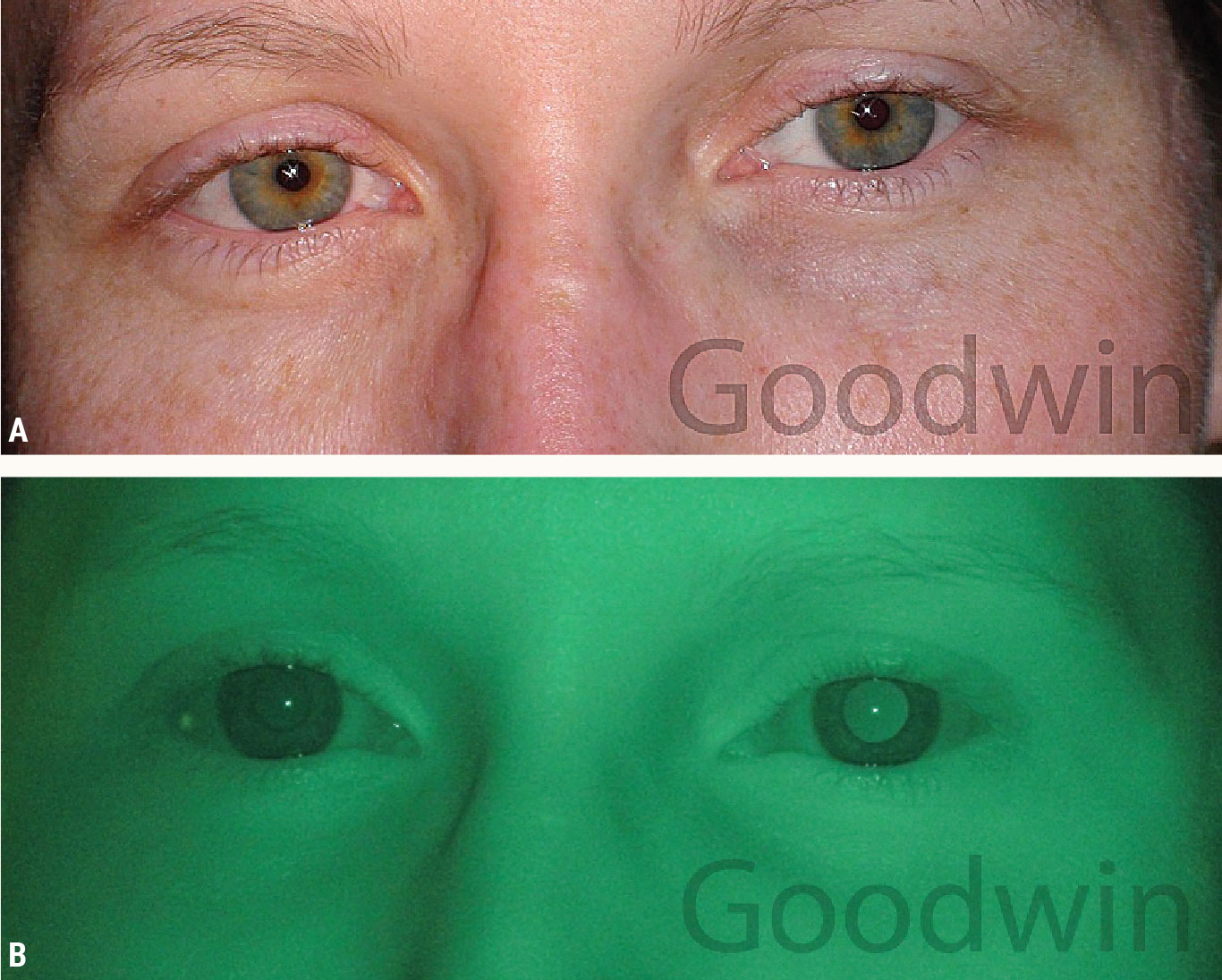 |
| Fig. 9. Physiologic anisocoria: (A) Anisocoria is evident in bright illumination, and both pupils respond briskly to light. (B) The anisocoria is slightly greater in dim illumination. Click image to enlarge. |
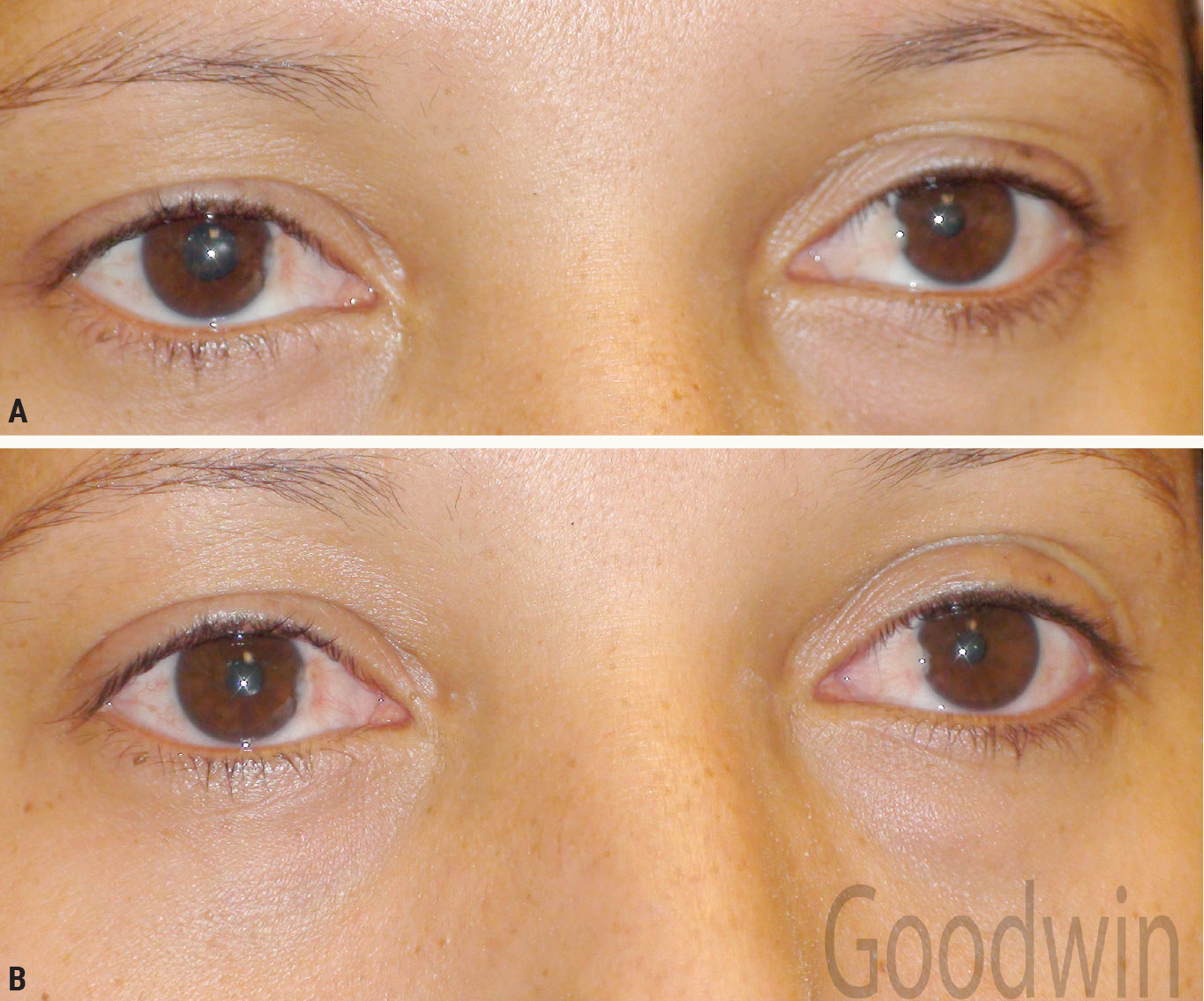
Horner syndrome. Figure 10 shows anisocoria greater in dim illumination. There is ptosis in the right eye, the eye with the smaller pupil. Pupil reactions to light are normal. The most distinguishing feature occurs when bright light is quickly removed from the eyes; the involved pupil dilates much slower than the other eye. This is called a dilation lag. This is most obvious by comparing the amount of anisocoria five seconds and 15 seconds after removing the light (Figure 10). With a sympathetic problem (Horner syndrome), the anisocoria will be greater after five seconds. A dilation lag will not occur with physiologic anisocoria.
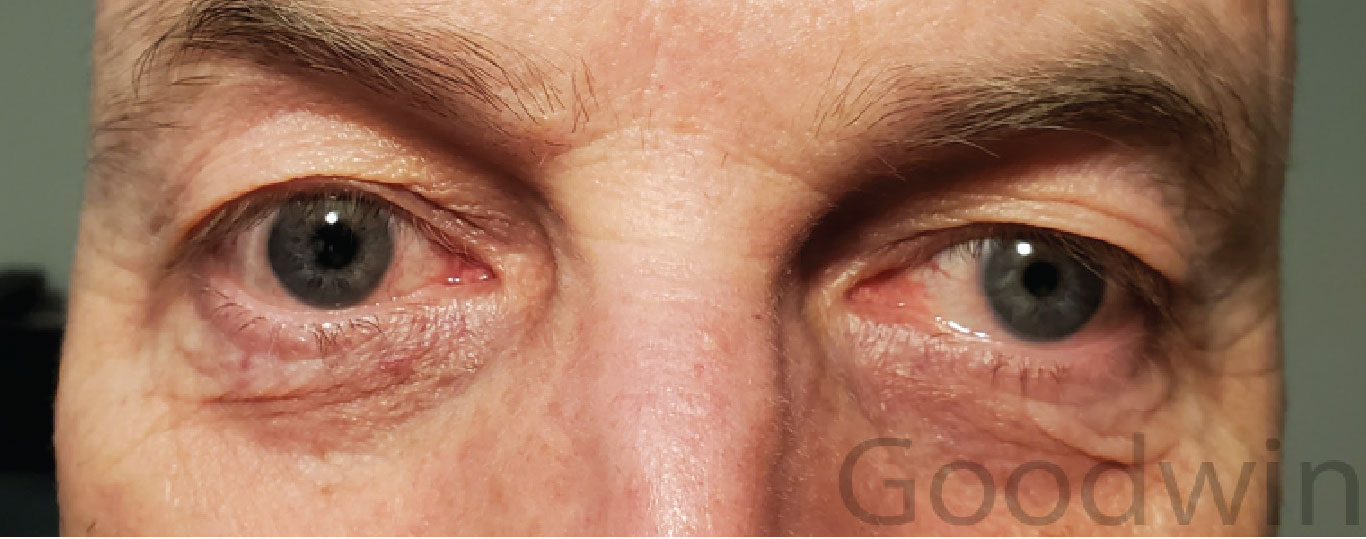
Characteristic signs of Horner syndrome include ptosis, miosis, anhidrosis and a dilation lag. Iris hypochromia will be present if the condition is congenital or longstanding (Figure 11). Hypochromia is never present in acute Horner syndrome.
Figure 11 shows a patient with a right preganglionic Horner syndrome. The left cheek is redder with sweat beads, indicating anhidrosis or lack of sweating on the right side. The distribution of the anhidrosis can give a clue to the lesion location. The sympathetic pathway starts in the hypothalamus. Fibers travel down the brainstem to the upper spinal cord where central fibers synapse with pre-ganglionic fibers. Preganglionic fibers exit the spinal cord, pass across the pulmonary apex and follow the carotid artery to the superior cervical ganglion. Postganglionic fibers start at the superior cervical ganglion, travel in the wall of the internal carotid artery to the cavernous sinus and then follow divisions of cranial nerves V and III to the pupil and Müller muscle.
While fibers to the pupil and eyelid follow the internal carotid artery, sweat fibers follow the external carotid artery. Therefore, a preganglionic lesion will cause loss of sweating to the entire half of the face. A person with a postganglionic lesion will have normal sweating on both sides of the face. This is true except for a small portion on the medial forehead, which is supplied by fibers following the internal carotid artery.
 |
Fig. 10. A right Horner syndrome: (A) In light, ptosis and miosis are evident in the right eye. (B) Five seconds after removing the light, there is significant anisocoria. (C) Fifteen seconds after removing the light, the anisocoria is less compared with that after five seconds. Click image to enlarge. |
Causes of Horner syndrome vary depending on whether the lesion involves the central, preganglionic or postganglionic fibers. Stroke, demyelination, trauma, malignancy of the lung or iatrogenic causes are common reasons for preganglionic Horner syndrome. The most emergent cause of Horner syndrome is an internal carotid artery dissection, which causes a postganglionic Horner syndrome.
Internal carotid artery dissection is a tear within the arterial wall. This results in a hematoma or clotting within the artery which can cut off blood flow to the brain. Internal carotid artery dissection can occur with trauma or spontaneously. Up to 58% of carotid artery dissections present with pain of the face, head or neck.3 Horner syndrome is present in 41% of carotid artery dissections.7 Carotid artery dissection is an emergency due to the very high risk of stroke. These patients need an emergent MRI and MRA of the head and neck. They’ll be admitted immediately and treated with anticoagulants.
Pharmacologic testing is necessary to ensure the patient actually has Horner syndrome. Physiologic anisocoria and ptosis by themselves are both very common, so the presence of both, even in the same eye, does not necessarily mean the patient has Horner syndrome. Apraclonidine will not dilate a normal pupil, but because iris dilator suprasensitivity occurs with Horner syndrome, it will dilate a Horner pupil (Figure 12).
Improvement of the ptosis also occurs. It takes a while to develop suprasensitivity after a nerve is damaged. Therefore, if the Horner syndrome is acute, apraclonidine will not work. Generally, it takes two to five days to develop suprasensitivity.5
Emergent Causes of Pupillary Disorders
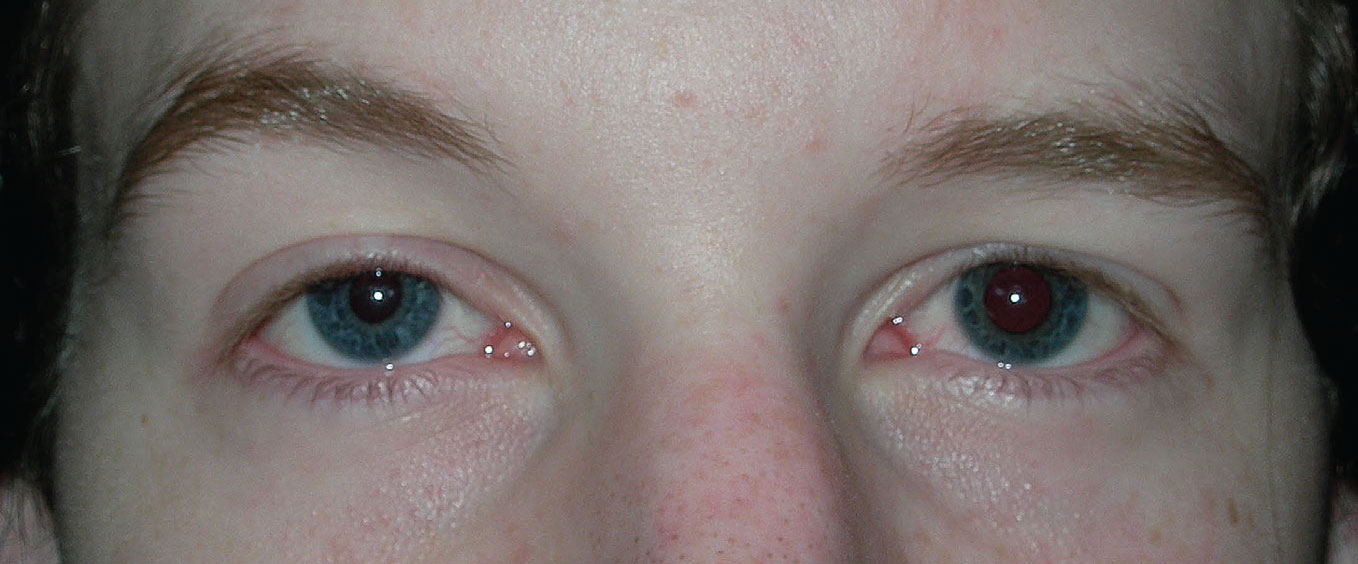 |
Fig. 11. Right Horner syndrome. The right pupil is smaller compared with the left, there is ptosis of the right eyelid and the right iris is of lighter color compared with the left iris. Click image to enlarge. |
Almost all true emergent conditions in optometry can be picked up by a pupil examination. With Horner syndrome, we are particularly worried about internal carotid artery dissection due to the risk of stroke. Both a cranial nerve III palsy involving the pupil and aberrant regeneration can indicate an aneurysm. A mid-dilated pupil with high intraocular pressure indicates angle-closure glaucoma.
The included flowchart with the emergent diagnoses only covered anisocoria, but an RAPD can be indicative of one of the most emergent conditions we deal with in ophthalmic disease: arteritic ischemic optic neuropathy. If you see an RAPD in a person over the age of 50, consider sending the patient for an emergent erythrocyte sedimentation rate and C-reactive protein to rule out giant cell arteritis. In addition, a central retinal artery occlusion or large retinal detachment, which are also emergencies, can cause an RAPD.
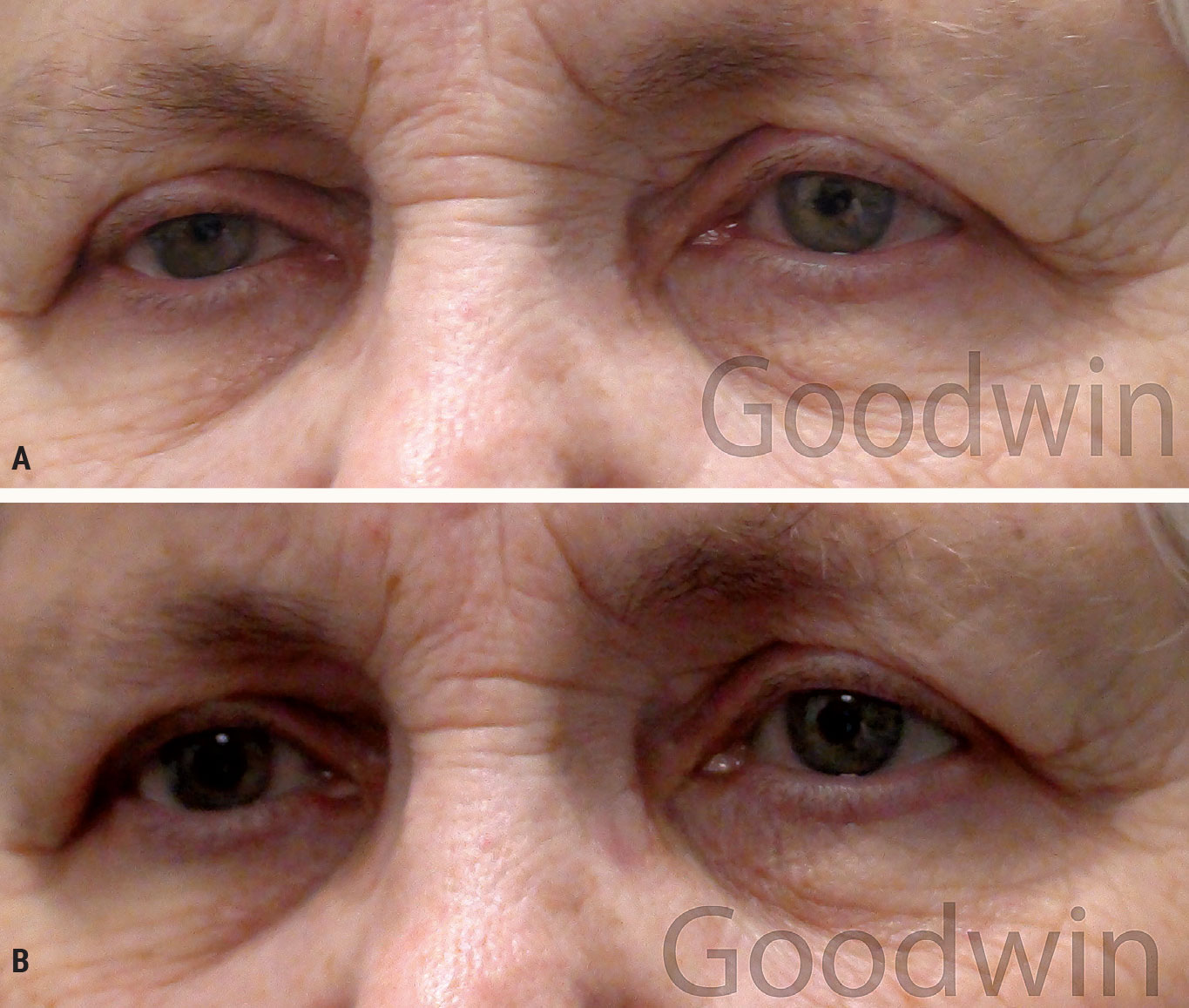 |
Fig. 12. Pharmacologic testing for Horner syndrome: (A) Before apraclonidine drops, miosis and ptosis are evident in the right eye. (B) After apraclonidine drops, there is reversal of the anisocoria (the right pupil is now larger than the left) and improvement of the ptosis. Click image to enlarge. |
Pupil abnormalities are commonly encountered by eyecare practitioners. Conducting a careful pupil examination can aid in recognizing and diagnosing important ocular and systemic conditions. While performing the pupil exam, keep in mind the most emergent causes of pupil abnormalities so that these are managed in a timely manner.
Dr. Goodwin is a professor at Pacific University College of Optometry, where she is also the coordinator of the neuro-ophthalmic disease referral service. In addition, she advises third-year students in a primary care clinic and works part-time in a private practice. She has no relevant financial interests to disclose.
1. Lam BL, Thompson, HS. An anisocoria produces a small relative afferent pupillary defect in the eye with the smaller pupil. J Neuroophthalmol. 1999;19(3):153-9. 2. Greenwald MJ, Folk ER. Afferent pupillary defects in amblyopia. J Pediatr Ophthalmol Strabismus. 1983;20(2):63-7. 3. Kardon R, Kawasaki A, Miller NR. Origin of the relative afferent pupillary defect in optic tract lesions. Ophthalmology. 2006;113(8):1345-53. 4. Hwang JM, Kim C, Kim JY. Relative afferent pupillary defect in patients with asymmetric cataracts. J Cataract Refract Surg. 2004;30(1):132-6. 5. Falardeau J. Anisocoria. International Ophthalmology Clinics Volume. 2019;59(3):125-39. 6. Steck R, Kong M, McCray K, et al. Physiologic anisocoria under various lighting conditions. Clin Ophthalmol. 2018;12:85-9. 7. Baumgartner R, Bogousslavsky J. Clinical manifestations of carotid dissection. Front Neurol Neurosci. 2005;20:70-6. 8. Remington L, Goodwin D. Clinical Anatomy and Physiology of the Visual System, 4th ed. Philadelphia, PA: Elsevier; 2021. |
