 |
What to Do When You See Uveitis
Here’s how to narrow down the differentials and determine when a case is—or isn’t—infectious.
By Megan Hunter, OD
Release Date: August 15, 2021
Expiration Date: August 15, 2024
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe the various causes of uveitis.
- Identify and diagnose a patient with uveitis.
- Determine if a case of uveitis is infectious or the result of a systemic disease.
- Discuss systemic etiologies of uveitis.
Target Audience: This activity is intended for optometrists engaged in uveitis management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Megan Hunter, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #122212 and course ID 73694-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Author: Dr. Hunter has no financial interests to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
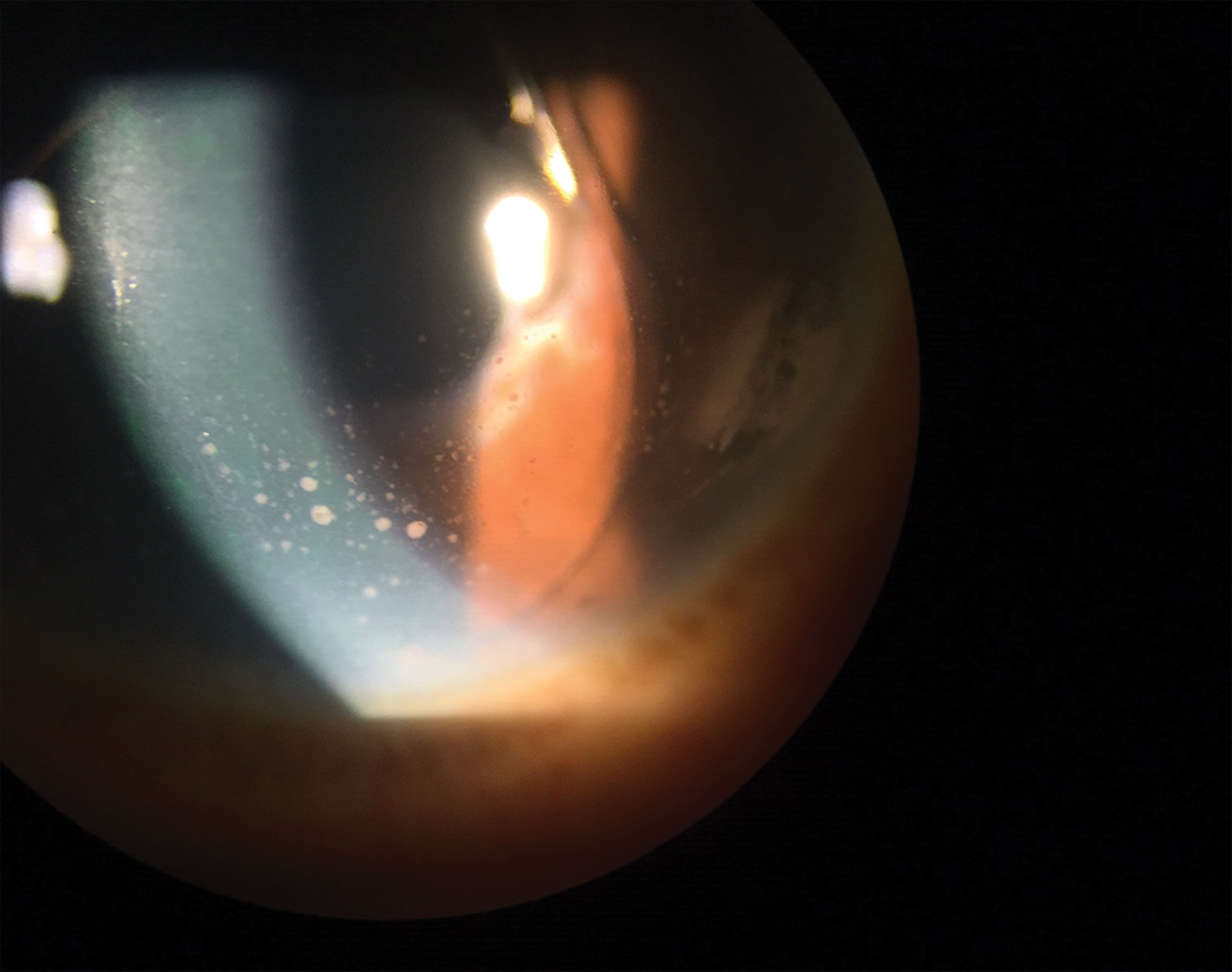 |
| Keratic precipitates are evident in this eye. Click image to enlarge. |
Uveitis—a threat to vision due to its potential to cause ocular complications—is responsible for 10% to 15% of severe visual handicap in the developed world and can affect all age groups.1,2 It stems from a wide array of conditions, both infectious and non-infectious. When a clinician sees a patient with uveitis, their differential list is long, and they must know how to narrow it down to arrive at the correct diagnosis; effective treatment depends on it.
The condition can be a result of systemic diseases such as HLA-B27 seronegative spondyloarthropathies, juvenile idiopathic arthritis, sarcoidosis and lupus, to name a few. It can also have infectious etiologies, such as herpes, syphilis, tuberculosis (TB) and Lyme disease.
This article will help clinicians wade through the differentials and better understand how to determine whether the uveitis is infectious, and, if not, how to reveal the systemic association. The location, onset and duration are key factors in the differential, as are the clinical findings that vary based on the underlying cause.
Clinical Features
The most common symptoms of anterior uveitis are pain, redness and severe photophobia. Patients may also experience tearing, blurred vision and floaters. Clinical signs of anterior uveitis include ciliary flush or circumlimbal injection, corneal edema and keratic precipitates (KPs). KPs are clumps of inflammatory cells that deposit on the corneal endothelium. They are more common on the inferior portion of the cornea. Fine KPs are referred to as nongranulomatous, and granulomatous KPs are greasy-appearing and made up of multiple cell clusters, including macrophages and giant cells.3
Anterior chamber cell and/or flare is a hallmark sign. In severe cases, the white inflammatory anterior chamber cells become layered and a hypopyon forms. Nodules are possible on the iris, and inflammation causes the iris to become sticky, leading to posterior synechiae and peripheral anterior synechiae. Vitreal signs of uveitis include vitreous cells, puffballs or snow banking and the accumulation of vitreous cells over the pars plana and peripheral retina. Posterior uveitis can be associated with additional inflammation in the posterior segment: cystoid macular edema, phlebitis, arteritis or disc edema.
The term uveitis is used to describe any inflammation along the uveal tract—the middle layer of the eye, which includes the iris, ciliary body and choroid. The word is not actually accurate regarding pathogenesis because other ocular structures are often the inflammatory target.4 The uvea transports more than 80% of the ocular blood volume, so it is regularly involved in intraocular inflammation.5
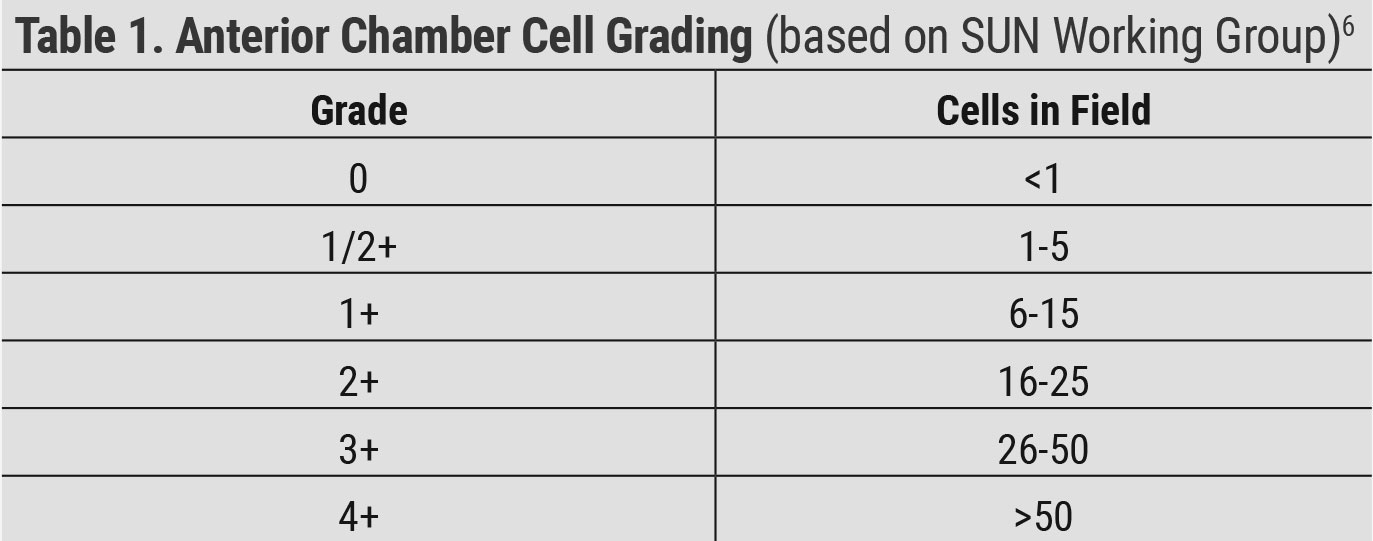 |
| Table 1. Anterior Chamber Cell Grading (based on SUN Working Group)6. Click table to enlarge. |
Uveitis is classified by location: anterior, intermediate, posterior or panuveitis. The latter term is used when there is no single predominant site of inflammation; rather, the condition is present in the anterior chamber, vitreous, retina and/or choroid.6 Posterior uveitis occurs when tissues that are typically protected by the blood-retinal barrier become affected. Examples include retinitis, retinal vasculitis, retinochoroiditis and optic neuritis.4 Cystoid macular edema can also occur in uveitis due to a disruption of the blood-retinal barrier.
Intraocular pressure (IOP) may be lower in patients with uveitis due to ciliary body inflammation, which leads to decreased aqueous production or higher due to trabeculitis characterized by debris and inflammatory cells, fibrin and edematous trabecular bands clogging the trabecular meshwork’s outflow channels.7
Angle closure is a possibility in uveitis. Inflammatory cells and fibrin can cause posterior synechiae, which may obstruct aqueous flow and lead to complete pupillary block. Chronic angle closure can occur when inflammatory cells and fibrin cause peripheral anterior synechiae through adhesions between the iris and trabecular meshwork. Furthermore, ciliary body inflammation and edema can cause it to rotate forward, closing the angle.
Examination and Diagnosis
When a patient comes in complaining of symptoms of anterior uveitis, a thorough ocular evaluation is important to make the diagnosis, discover the etiology and gauge the response to treatment. A key step includes recording visual acuity. A careful slit lamp examination is a must to assess the cornea for any KPs, followed by a careful examination of the anterior chamber for inflammatory cells or protein (flare).
Inflammatory cells in the anterior chamber are a result of spillover from the inflammation in the iris and/or ciliary body. Anterior chamber cells are best detected in a dark room with a bright slit lamp beam with higher magnification obliquely directed through the aqueous.
The Standardization of Uveitis Nomenclature (SUN) Project was started by a group of uveitis specialists to develop criteria to standardize the reporting of uveitis in the literature and at academic meetings. The group met for the first time in 2004 and first published standard reporting guidelines in 2005.6 They determined how to grade anterior chamber cells based on the amount of cells seen in a 1mm x 1mm slit beam and established criteria for the grading of anterior chamber flare (Tables 1 and 2). It is important to measure IOP and check gonioscopy if pressure is elevated. A dilated fundus examination is crucial to look for vitreous and posterior segment inflammation.
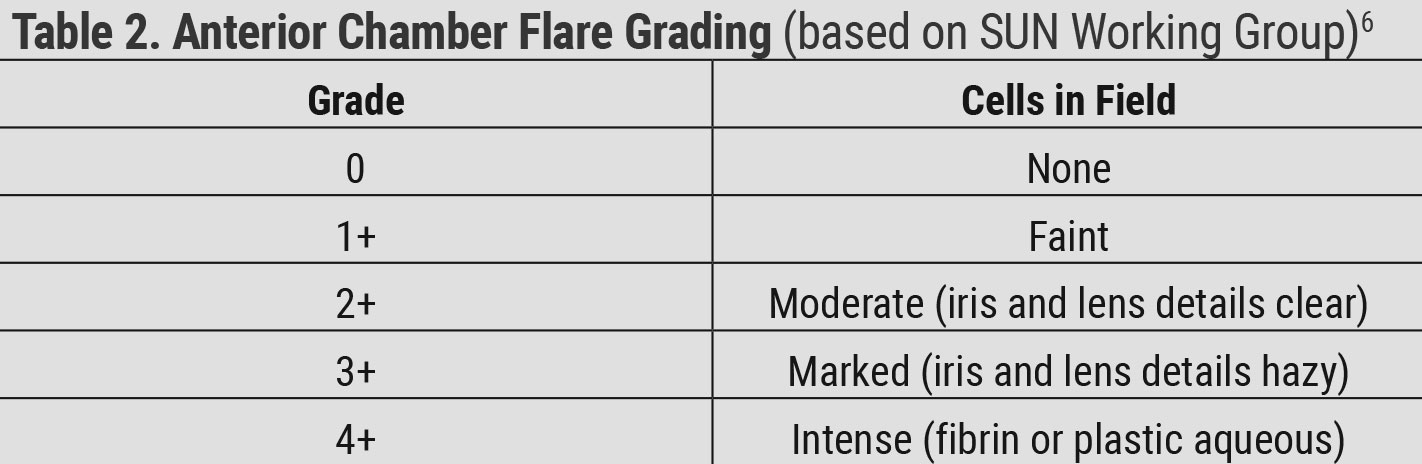 |
| Table 2. Anterior Chamber Flare Grading (based on SUN Working Group)6. Click table to enlarge. |
Underlying Etiology
After making the diagnosis of uveitis, it is important to start considering the underlying etiology. Detailed history and examination as well as imaging studies and laboratory testing, when indicated, help determine the underlying systemic condition if there is one. Most importantly, the clinician must determine if the uveitis is of infectious origin and caused by one of the following underlying etiologies:
Syphilis. A systemic infection caused by the spirochete bacterium Treponema pallidum, syphilis is almost exclusively spread by sexual contact.8 Syphilis is on the rise, and its incidence has been increasing across many groups in the United States.8 It has been called the “great imitator” due to its multiple ocular presentations and, therefore, its ability to mimic many ocular diseases.
Posterior uveitis and panuveitis are the most common syphilitic presentations of uveitis. There are three stages of systemic syphilis infection: primary, secondary and tertiary. Primary syphilis is characterized by a chancre, which is a painless erythematous ulcer seen on the anus, mouth, penis or vagina. It appears approximately 21 days after exposure to Treponema pallidum and heals in one to two months even if untreated. The fluid produced from a chancre is very infectious. Ocular syphilis is almost nonexistent in the primary stage.9
Secondary syphilis develops four to 10 weeks after the initial infection and is characterized by a body maculopapular or pustular rash most common on flexor and volar surfaces. Other secondary syphilis signs are fever, malaise, headache, nausea, loss of appetite and joint pain. Ocular involvement is possible in the secondary stage and may present as keratitis, iridocyclitis, episcleritis, scleritis, chorioretinitis or vitritis.8 The latent stage begins one year after infection, with most people remaining in this stage. A patient with latent syphilis will have positive serology but no clinical signs or symptoms of infection.
Roughly 15% to 30% of infected individuals who do not get treatment will develop tertiary syphilis.10 This is a destructive immune response to the low levels of remaining Treponema pallidum. Tertiary syphilis is characterized by benign gummas under the skin but can cause skeletal, heart and blood vessel damage. It typically appears three to 15 years after initial infection.
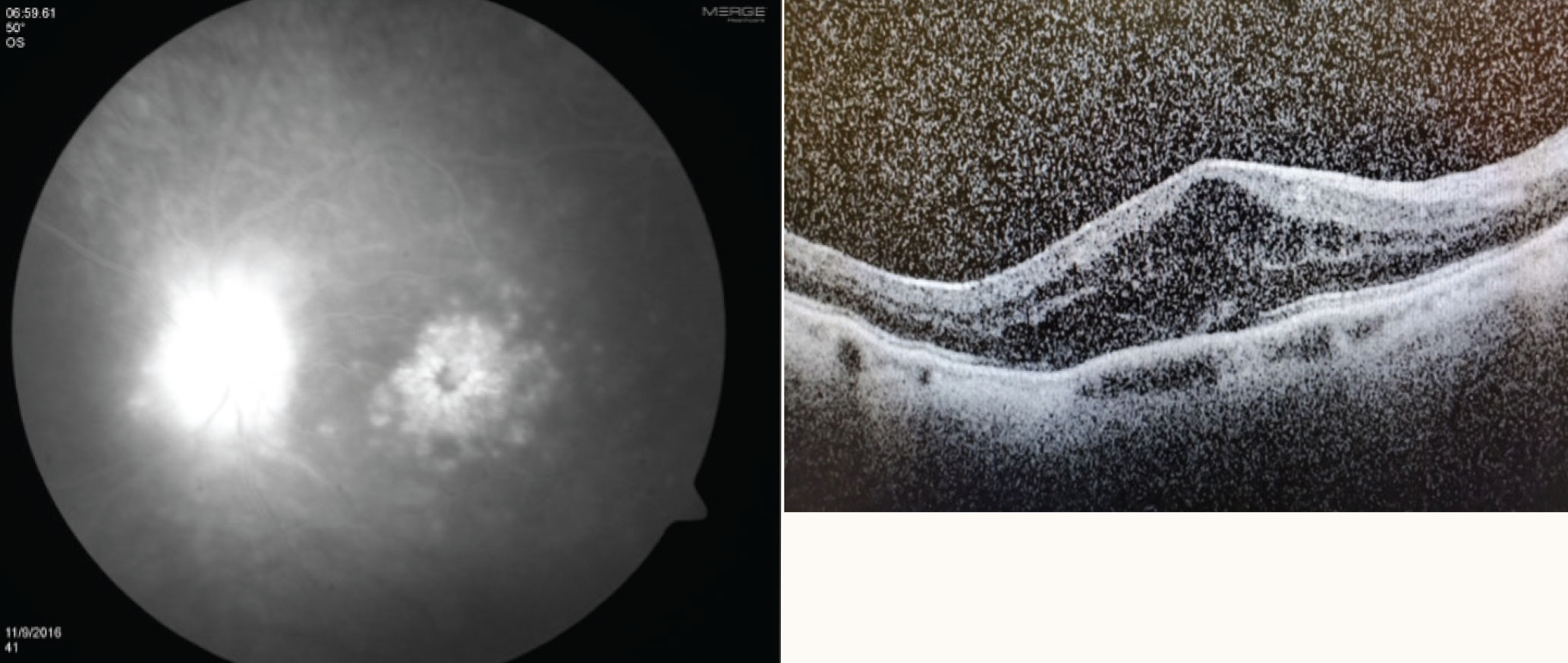 |
| Cystoid macular edema on fluorescein angiography (left) and optical coherence tomography (right). Click image to enlarge. |
Ocular syphilis occurs more commonly in the latent and tertiary stages.9 In latent syphilis, it can be the only presenting sign.9
Uveitis caused by syphilis can be granulomatous or non-granulomatous, unilateral or bilateral and anterior, intermediate, posterior or panuveitic.11 Chorioretinitis is the most common presentation of syphilitic posterior uveitis and is often accompanied by vitritis and characterized by grayish-yellow choroidal lesions in the posterior pole and mid-periphery.12 Focal retinal edema, vasculitis and papillitis are all signs of syphilitic uveitis, as well as necrotizing retinitis, retinal vasculitis and exudative retinal detachment. Neurosyphilis is characterized by aseptic meningitis with cranial nerve palsies, Argyll Robertson pupil (small pupils that react to accommodation but not to light), progressive loss of cortical function and tabes dorsalis (degeneration of the dorsal root ganglia of the spinal cord causing pain, ataxia, paresthesias, hypoesthesias and decreased deep tendon reflexes).
Diagnosis of syphilis is commonly made through serologic testing, of which there are two categories: nontreponemal (regain) tests and treponemal-specific tests. Nontreponemal tests (rapid plasma regain and venereal disease research laboratory) detect nonspecific treponemal antibody. Treponemal-specific tests (fluorescent treponemal antibody absorption and micro-hemagglutination treponemal pallidum) detect specific treponemal antibody.
Nontreponemal tests are used as screening tests but also successfully monitor response to treatment and disease activity. A four-fold change in titer or two dilutions represents a successful response to treatment. High titers may not decrease for 12 to 24 months following treatment.
Treponemal tests are more specific and are used to confirm the diagnosis after a positive nontreponemal test. They have a lower percentage of false positive results.
Nontreponemal tests become positive within three weeks of primary infection, so a negative result may represent a very early infection.13 A negative test result when there is a high index of suspicion should be repeated in two to three weeks. Both types of tests—nontreponemal and treponemal— are needed to confirm a diagnosis of syphilis.14
Syphilis is treated with penicillin G with dose and type of administration depending on the stage of disease. In primary, secondary and early latent syphilis, one intramuscular administration of 2.4 million units is sufficient treatment. In latent syphilis of unknown duration, late latent syphilis and tertiary syphilis, three intramuscular doses are given at one-week intervals. For neurosyphilis cases, three to four million units are given intravenously every four hours for 10 to 14 days. The CDC recommends a lumbar puncture to check for neurosyphilis in all patients with ocular syphilis.15 Ocular syphilis is treated the same as neurosyphilis even with a normal cerebrospinal fluid study.
It is always wise to consider syphilis in any case of uveitis. Ask about sexual activity and prior presence of a chancre or body rash. According to uveitis specialist Janet Davis, “For most incident cases of uveitis, a strict rule of always placing syphilis in the differential diagnosis and including treponemal serological testing in any laboratory work-up is wise.”11
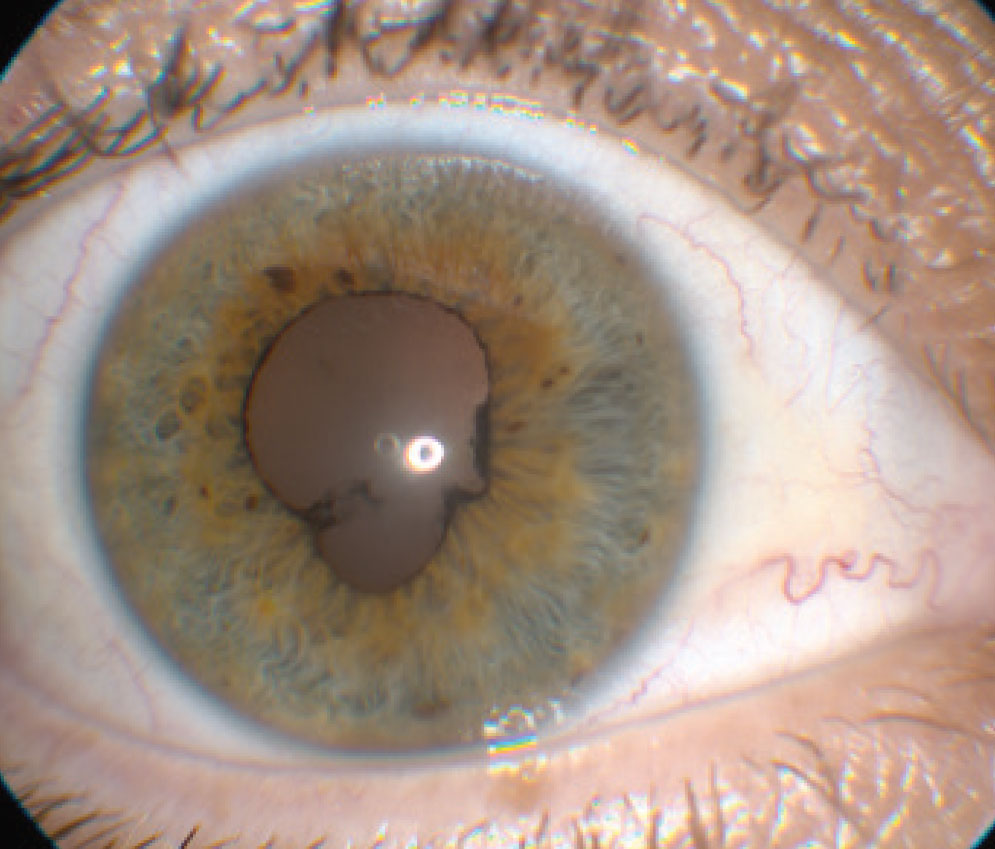 |
| This patient has posterior synechiae. Click image to enlarge. |
TB. This condition—caused by infection from Mycobacterium TB—is transmitted by aerosolized droplets. It is important to note that exposure is not equivalent to active infection. Most patients develop a self-limiting pneumonia that heals with calcified granuloma formation that can later reactivate if the patient becomes immunosuppressed. Signs of active infection are coughing, malaise, fever, night sweats and weight loss.
Incidence has decreased dramatically in the United States, but TB infection remains a concern due to immunocompromise and immigration.16 Most TB cases in the United States occur in foreign-born individuals likely due to reactivation of latent infection acquired prior to arriving in the country, most commonly from Mexico, the Philippines, India, Vietnam and China.16 Other risk factors for TB infection are human immunodeficiency virus (HIV) coinfection, diabetes, excessive alcohol abuse, drug use, homelessness and incarceration.
Intraocular tuberculosis (IOTB) is a form of extrapulmonary TB and is caused by either hypersensitivity to the tubercular antigen or by tubercle bacilli directly invading the eye. A primary pulmonary source is often not found in IOTB as is the case in other forms of extrapulmonary TB.17 Posterior uveitis is the most common manifestation of IOTB, and the choroid is commonly the involved structure, resulting in choroidal tuberculomas, multifocal choroiditis or serpiginous-like choroiditis.18 Choroidal tubercles are grayish-white/yellow in color and can be unilateral or bilateral and single lesions or multiple. Large, single choroidal TB granulomas can cause exudative retinal detachments.
TB serpiginous-like choroiditis occurs in younger patients and is often bilateral and associated with significant vitritis. Anterior segment inflammation is common. This condition begins as multifocal yellowish-white lesions that are initially discreet but become confluent over a few weeks.19 Alternatively, this condition may present as a placoid pattern with a large plaque-like lesion with a leading edge that is yellowish-white and elevated with a flatter and pigmented center.19 Tubercular serpiginous-like choroiditis is a distinct entity common in younger adults from Asian-Pacific regions where TB is endemic.18-20 Other, less common signs of ocular TB are conjunctivitis, conjunctival nodules, interstitial and phlyctenular conjunctivitis, anterior uveitis, episcleritis, scleritis, retinal perivasculitis and optic neuritis.
Work-up for the patient with suspected tuberculosis includes a chest X-ray to look for active pulmonary involvement and either a TB skin test (PPD) or interferon gamma release assay (IGRA) blood test (QuantiFeron-TB Gold, T-SPOT TB test). The skin test injects purified protein derivative intradermally. TB-infected patients will show a delayed hypersensitivity reaction within six weeks of active infection, which will remain positive for life.
The skin test must be read 48 to 72 hours after injection of the purified protein derivative; this requires a return to clinic. The IGRA blood tests have become more popular in the work-up of TB because results are available in 24 hours and do not require a second visit. Both TB skin tests and IGRA blood tests are accurate in the detection of latent infection, but both are not accurately able to predict risk for progression from latent infection to active infection.21 Confirmation of pulmonary TB requires microbiological evidence of Mycobacterium TB from sputum.
Confirming the diagnosis of intraocular TB is tough and requires microbiological confirmation of Mycobacterium TB from ocular fluid through PCR testing of aqueous or vitreous tap. Due to the invasive nature of the test, it has yet to be routinely adopted. Intraocular TB is often presumed in the presence of at least one clinical sign of the condition, signs of confirmation of pulmonary TB, immunologic evidence of TB infection or documented exposure.22
Treatment of TB includes a combination of four drugs: rifampin, isoniazid, pyrazinamide and ethambutol (RIPE therapy). Patients on ethambutol must be monitored for ocular toxicity due to the risk of ethambutol-induced optic neuropathy. RIPE therapy has been shown to be effective against intraocular TB due to the treatment of latent TB infection of the body decreasing the hypersensitivity reaction in the eye.23,24
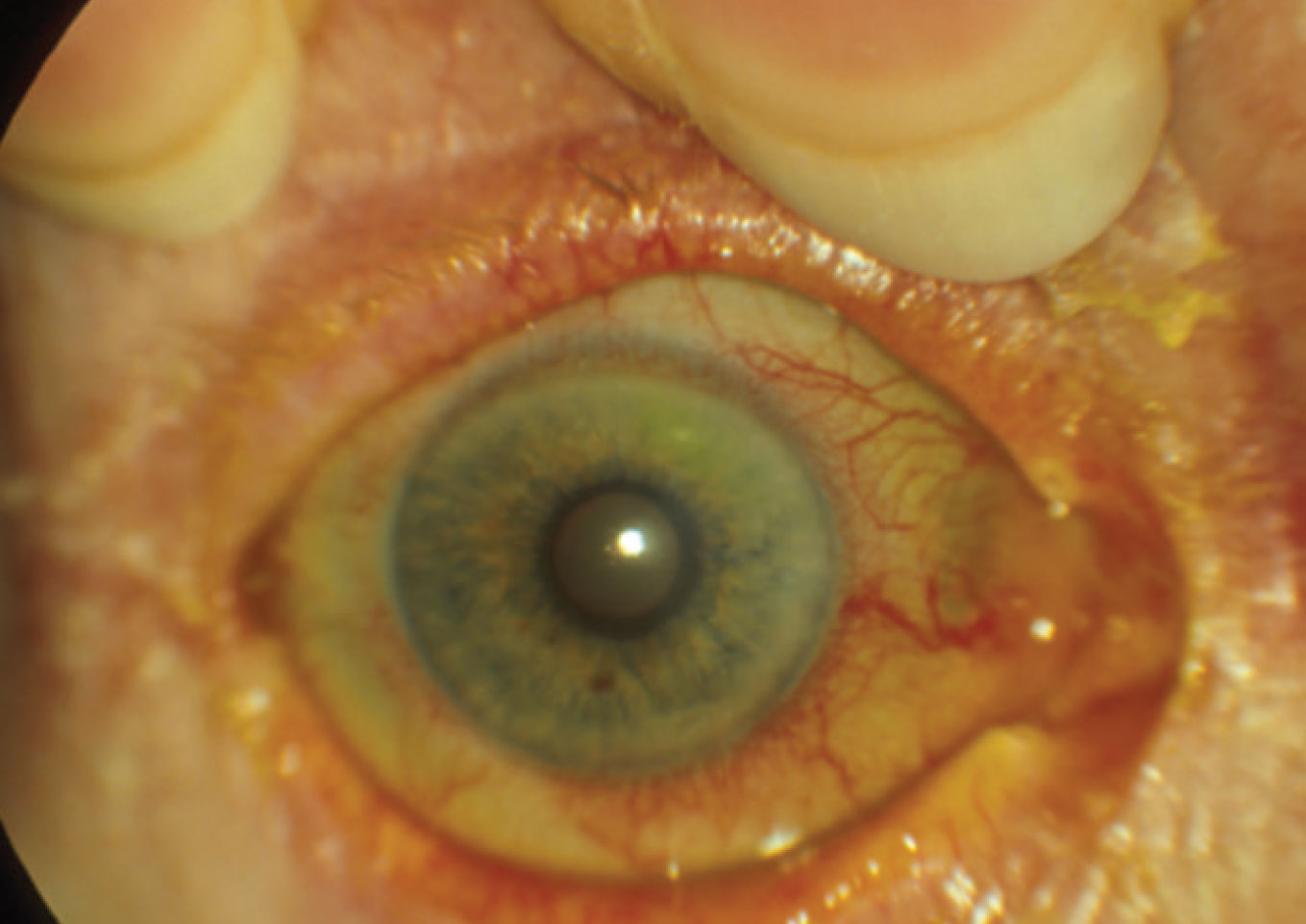 |
| A ciliary flush is visible on examination. Click image to enlarge. |
Toxoplasmosis. Ocular toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii. Humans catch this parasite by eating undercooked meat or by hand contamination when cleaning cat litter boxes and transferring the germs onto food. Children can become infected by eating dirt that contains spore of the parasite. In endemic areas, water contamination aids in transmission of the disease.9
Clinical manifestation of active disease is a focal gray-white lesion of retinal necrosis, which typically develops on the edge of a preexisting pigmented chorioretinal scar. Active toxoplasmosis infection is associated with a dense vitritis. Patients are at lifetime risk of recurrence because tissue cysts remain in the retina.25 Treatment of active lesions includes antibiotics and corticosteroids. The most frequent treatment regimen is a pyrimethamine-sulfadiazine combination in addition to steroids.9 Prophylactic antibiotic treatment has recently been proposed.25
Lyme disease. This is caused by the spirochete Borrelia burgdorferi and leads to a multisystem infectious response. It is spread by certain species of ticks. Symptoms include erythema, migraines, fever, headache and fatigue. If untreated, infection can lead to arthritis, neurological manifestations and cardiac involvement. Ocular findings are rare and occur in less than 1% of cases.26
Lyme can cause all types of uveitis but it is very rare, accounting for less than 1% of cases.27 The CDC recommends serological testing in symptomatic patients with a risk of exposure to black-legged ticks.28 In July 2019, the FDA cleared serologic assays that use enzyme immunoassay in a two-test format in place of the confirmatory western immunoblot assay as the second test.29 Lyme disease is treated with antibiotics, and those treated in the early stages of disease recover completely.
Viral uveitis. The most common viruses associated with anterior uveitis are herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV) and rubella virus (RV).30 Clinical features of viral uveitis include KPs, elevated IOP and iris atrophy. Viral uveitis has a spectrum of clinical presentations, and different viruses cause similar presentations of uveitis, making it difficult to determine the causative virus.
Fuchs’ uveitis syndrome is usually unilateral and has a mild anterior chamber reaction, no ciliary injection, KPs, heterochromia, vitritis and posterior subcapsular cataract. The affected eye is typically lighter in color due to pigment loss but can appear darker in brown eyes due to anterior stromal atrophy, which reveals the darker pigmented epithelium.31 Structural iris changes are common with atrophy, affecting all layers and causing the loss of the corrugated texture.31
Fuchs’ likely represents a low-grade immunoreactivity to viral antigen. RV is the most common causative virus in the United States and Europe, with rubella-specific antibodies detected in the aqueous humor of eyes diagnosed with Fuchs’ uveitis syndrome.32,33 This disease is less common in patients born in the United States after initiation of the rubella vaccination program.34 CMV is the most common cause of Fuchs’ uveitis syndrome in East Asia.35
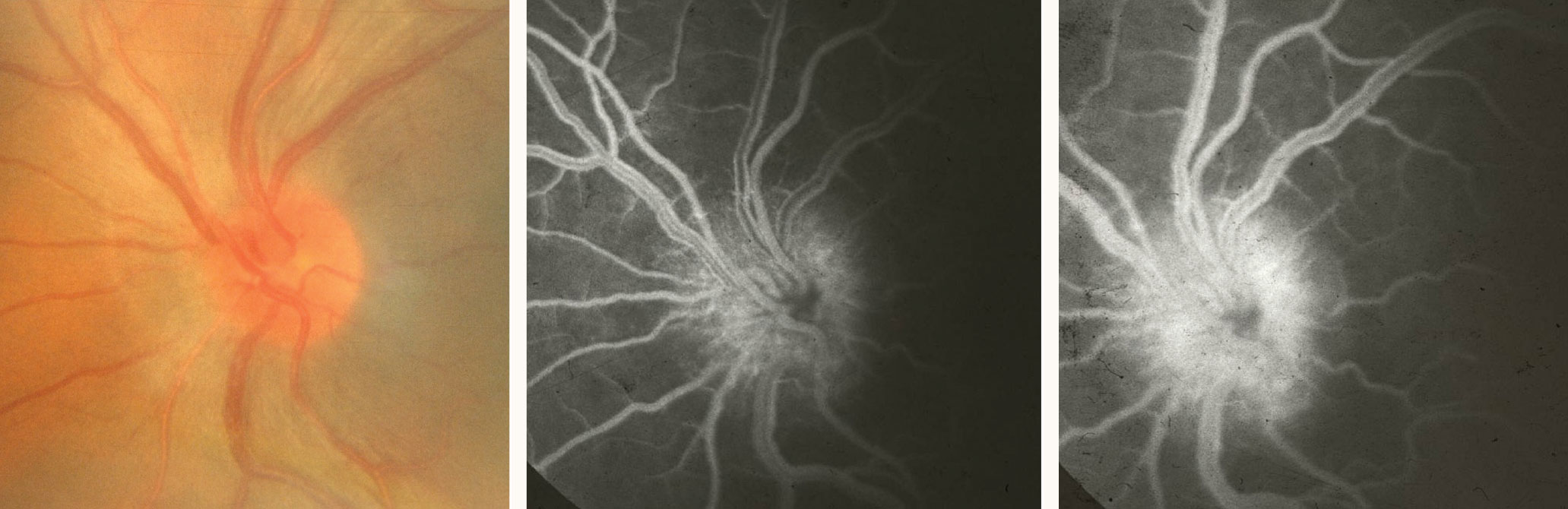 |
| Pictured here is an HLA-B27–positive uveitis patient. Optic nerve head edema is evident on fundus photography (left), early fluorescein angiography (middle) and late fluorescein angiography (right). Click image to enlarge. |
Posner-Schlossman syndrome (PSS) is associated with recurrent attacks of elevated IOP and corneal endotheliitis manifested by corneal edema, mild anterior chamber reaction and a few KPs. In endotheliitis, the corneal endothelium is thought to be the primary inflammatory source. IOP is markedly high (mean 48mm Hg to 50mm Hg), which can cause mild discomfort, mild visual blur and the presence of halos alerting the patient to seek eye care.35 CMV-specific antibodies have been detected in the aqueous of patients diagnosed with PSS, with CMV appearing to be a frequent cause of PSS.35,36 Treatment includes ocular hypotensive medication and topical steroids to control the inflammation and help lower IOP.
HSV anterior uveitis occurs more often during reactivation of the virus than during the primary infection.30 The HSV virus lays dormant in the trigeminal ganglion, and its reactivation can cause intraocular inflammation. Clinical signs of HSV anterior uveitis include elevated IOP due to trabeculitis, moderate anterior chamber reaction and ciliary flush. Vitritis and posterior synechiae are possible clinical features. Iris involvement leads to loss of pigment epithelial cells with iris atrophy and transillumination defects in up to 50% of affected individuals.37
Ask patients about a history of frequent cold sores or previous keratitis. Corneal scars and decreased corneal sensitivity may be present from previous infection. HSV anterior uveitis is more common in patients with active corneal infection or a history of prior keratitis.38 Dendritic keratitis is the hallmark presentation of epithelial keratitis from HSV.
Anterior uveitis from HSV is treated with oral antiviral medications, topical prednisolone acetate every one to two hours and a cycloplegic agent to relax the ciliary body.39 When considering an antiviral, oral medications are necessary to penetrate the anterior chamber, and topical antivirals are eventually toxic to the cornea. Oral antiviral options are 400mg of acyclovir five times daily or 500mg of valacyclovir three times daily. A history of epithelial disease can accompany HSV anterior uveitis. Prophylactic antiviral therapy to prevent epithelial recurrence is important and dosed at 400mg of acyclovir two times daily or 500mg to 1,000mg of valacyclovir once daily.
VZV lies dormant in the neural sensory ganglia after primary infection. It can reactivate after immunity wanes, which is common later in life.30 Herpes zoster ophthalmicus (HZO) presents with pain and a vesicular skin rash along the ophthalmic division of the trigeminal nerve. Some patients have only the skin rash in HZO, but anterior uveitis can develop as well. The uveitis that arises from VZV is often more severe than that of HSV and is accompanied by a higher incidence of synechiae and vitritis. Corneal pseudodendrites are seen in HZO, which are elevated but lack terminal bulbs.
HZO is treated with acyclovir 800mg five times daily or valacyclovir 1,000mg three times daily. Topical prednisolone acetate and cycloplegic agents are used to control the anterior chamber inflammation. IOP-lowering medications are utilized to help control pressure in patients with viral uveitis, although controlling the inflammation also helps manage the eye pressure. Prostaglandin analogs should be avoided due to their potential to contribute to inflammation.
Viruses that are emerging as causes of uveitis are dengue virus and chikungunya virus. Dengue is spread through mosquitos, and while rare in the United States, it is more common in Southeast Asian countries and is on the rise in the Caribbean.40 Most reported cases in the United States have been associated with recent travel.
Dengue fever symptoms include high fever, headache, vomiting, joint pain and rash. Common ocular manifestations are chorioretinitis with macular edema, foveolitis and periphlebitis.41 Foveolitis is the presence of well-defined, yellow subretinal lesions in the macula accompanied by retinal striae.42 Ocular associations can improve without intervention, although some patients need treatment with systemic steroids to control inflammation.
Chikungunya is also spread through mosquito bites and is endemic in Asia and Africa.20 Infection causes fever and joint pain. Common ocular signs of chikungunya infection are conjunctivitis, anterior uveitis, dendritic keratitis and increased IOP. Treatment of chikungunya is supportive in nature, including the ocular inflammation which is treated with steroids and cycloplegic agents.
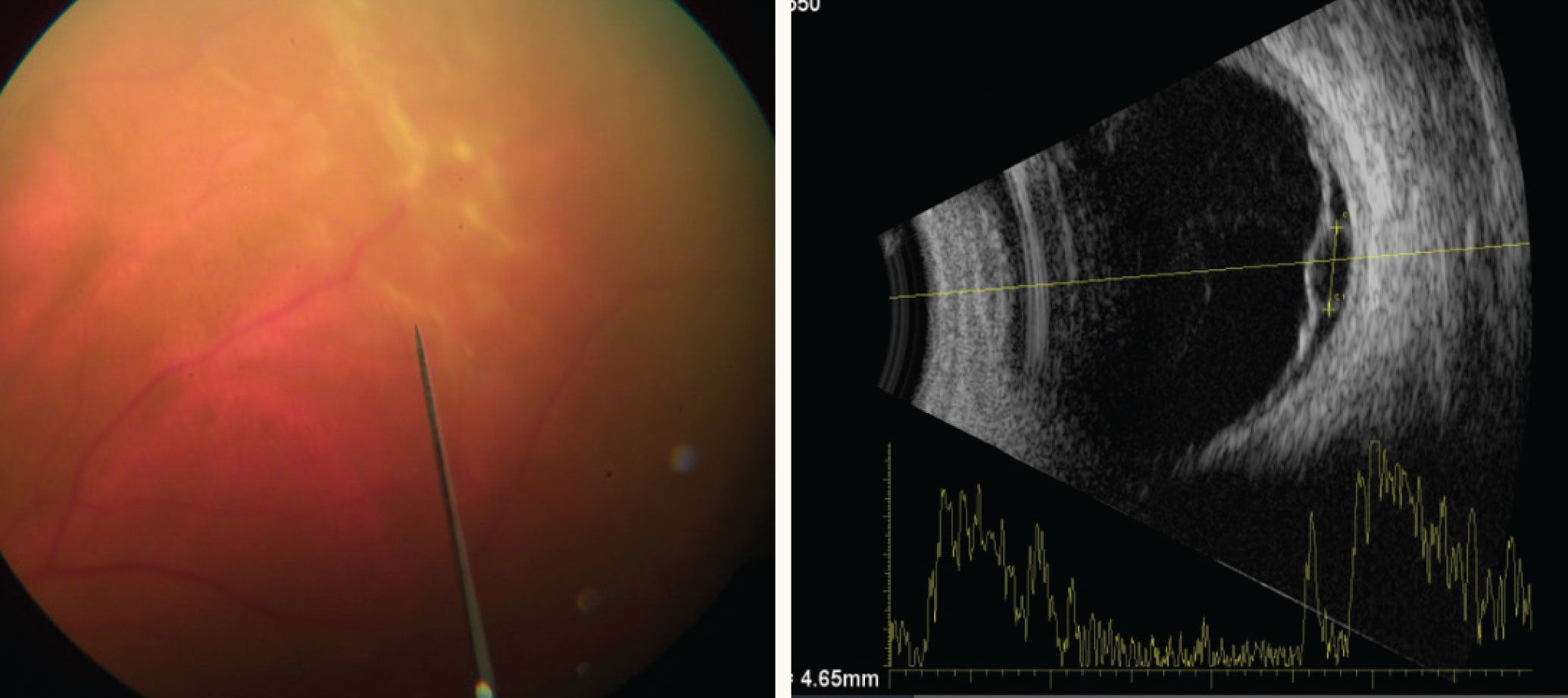 |
| Retinal detachment in a patient with a solitary tubercular granuloma (left) on B-scan ultrasonography (right). Click image to enlarge. |
CMV retinitis. In immunocompetent people, CMV is associated with Fuchs’ uveitis syndrome and PSS. CMV retinitis is the most common opportunistic infection in the immunosuppressed and much more devastating for these individuals.9 Active CMV retinitis can be hemorrhagic with white/yellow retinal lesions, granular with no necrosis or hemorrhage or perivascular with white lesions surrounding the blood vessels. Early CMV retinitis can resemble large cotton wool spots. Treatment options include intravenous, intravitreal and oral antiviral medications.
Non-infectious cases. A common cause of non-infectious, immune-mediated posterior uveitis is sarcoidosis. This is a multisystem inflammatory disorder characterized by non-caseating granulomas. Sarcoidosis can involve any organ or system but most commonly affects the lungs, skin and reticuloendothelial system (liver, spleen and lymph nodes).43 Taking a chest X-ray, conducting liver enzyme blood testing and asking about skin lesions is a good investigational approach.43
Pulmonary sarcoidosis is diagnosed by the detection of bilateral hilar adenopathy on a chest X-ray. Lysozyme- and angiotensin-converting enzymes are released by granulomas; therefore, blood tests that aid in granuloma detection are often ordered in the work-up of a uveitis patient, even though they have a low positive predictive value.43 Common ocular signs of sarcoidosis include vitritis, periphlebitis, multifocal choroiditis and papillitis.
A detailed case history can help separate the possibility of syphilis, TB or sarcoidosis as the etiology of posterior uveitis. Ask about sexual habits, HIV status, country of origin and history of previous lung infection, chancre or body rash.
The most common anterior uveitis in the United States is not infectious but rather immune-mediated HLA-B27-associated uveitis.30 This is typically unilateral and acute, associated with marked fibrinous anterior chamber reaction with hypopyon. IOP is often lower due to reduced aqueous production caused by ciliary body inflammation.
Conclusion
A detailed case history and careful examination are key elements of our clinical regimen when tasked with the diagnosis and management of uveitis. It is important to rule out infectious causes of uveitis so that necessary systemic therapy can be initiated. In cases of posterior uveitis, consider the possibility of syphilis or tuberculosis. In cases of anterior uveitis, consider a viral origin whenever IOP is elevated or iris atrophy is present.
Dr. Hunter graduated from the Illinois College of Optometry in 2002 and completed a residency in ocular disease at the Bascom Palmer Eye Institute in 2003. She is currently an associate professor of ophthalmology at the Loyola University Medical Center and the Hines VA Medical Center. She has no financial interests to disclose.
1. Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123(3):655-62. 2. Miserocchi E, Fogliato G, Modorati G, et al. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705-17. 3. Chan NS, Chee SP. Keratic precipitates: the underutilized diagnostic clue. Ocul Immunol Inflamm. 2021:1-10. 4. Forrester JV, Kuffova L, Dick AD. Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol. 2018;189:77-85. 5. Forrester JV, Dick AD, McMamin P, et al. The Eye – Basic Science and Practice. 4th ed. London: Elsevier; 2015. 6. Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-16. 7. Kesav N, Palestine AG, Yahook MY, et al. Current management of uveitis-associated ocular hypertension and glaucoma. Surv Ophthalmol. 2020:65(4):397-407. 8. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019. www.cdc.gov/std/statistics/2019/default.htm. Accessed July 29, 2021. 9. Majumder PD, Ghosh A, Biswas J. Infectious uveitis: an enigma. Middle East Afr J Ophthalmol. 2017;24(1):2-10. 10. Radhakrishnan R. MedicineNet. What happens if syphilis is left untreated? August 26, 2020. www.medicinenet.com/what_happens_if_syphilis_is_left_untreated/article.htm. Accessed July 29, 2021. 11. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-8. 12. Koundanya VV, Tripathy K. StatPearls [Internet]. Syphilis ocular manifestations. February 14, 2021. www.ncbi.nlm.nih.gov/books/NBK558957/. Accessed July 29, 2021. 13. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86(5):433-40. 14. Centers for Disease Control and Prevention. Syphilis – CDC fact sheet. www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Accessed July 29, 2021. 15. Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. June 5, 2015. www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm. Accessed July 29, 2021. 16. Centers for Disease Control and Prevention. Trends in tuberculosis, 2019. www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm. Accessed July 29, 2021. 17. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis – an update. Surv Ophthalmol. 2007;52(6):561-87. 18. Gupta V, Shoughy SS, Mahajan S, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):14-24. 19. Bansal R, Gupta A, Gupta V, et al. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119(11):2334-42. 20. Agarwal A, Aggarwal K, Gupta V. Infectious uveitis: an Asian perspective. Eye (Lond). 2019;33(1):50-65. 21. Trajman A, Steffen RE, Menzies D. Interferon-gamma release assays versus tuberculin skin testing for the diagnosis of latent tuberculosis infection: an overview of the evidence. Pulm Med. 2013;2013:601737. 22. Gupta A, Sharma A, Bansal R, et al. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):7-13. 23. Bansal R, Gupta A, Gupta V, et al. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol. 2008;146(5):772-9. 24. Agrawal R, Gupta B, Gonzalez-Lopez JJ, et al. The role of anti-tubercular therapy in patients with presumed ocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):40-6. 25. Reich M, Mackensen F. Ocular toxoplasmosis: background and evidence for antibiotic prophylaxis. Curr Opin Ophthalmol. 2015;26(6):498-505. 26. Weinberg RS. AAO. Ocular involvement in Lyme disease. November 5, 2008. www.aao.org/current-insight/ocular-involvement-in-lyme-disease. Accessed July 29, 2021. 27. Rosenbaum JT, Rahn DW. Prevalence of Lyme disease among patients with uveitis. Am J Ophthalmol. 1991;112(4):462-3. 28. Centers for Disease Control and Prevention. Lyme disease. www.cdc.gov/lyme. Accessed July 29, 2021. 29. Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. 30. Chan NSW, Chee SP. Demystifying viral anterior uveitis: a review. Clin Exp Ophthalmol. 2019;47(3):320-33. 31. Jones NP. Fuchs’ heterochromic uveitis: a reappraisal of the clinical spectrum. Eye (Lond). 1991;5:649-61. 32. Quentin CD, Reiber H. Fuchs heterochromic cyclitis: rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol. 2004;138(1):46-54. 33. Rothova A. The riddle of Fuchs heterochromic uveitis. Am J Ophthalmol. 2007;144(3):447-8. 34. Birnbaum AD, Tessler HH, Schultz KL, et al. Epidemiologic relationship between Fuchs heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol. 2007;144(3):424-8. 35. Chee SP, Jap A. Presumed Fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol. 2008;146(6):883-9. 36. Bloch-Michel E, Dussaix E, Cerqueti P, et al. Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann syndrome. Int Ophthalmol. 1987;11(2):95-6. 37. Tabbara KF, Chavis PS. Herpes simplex anterior uveitis. Int Ophthalmol Clin. 1998;38(4):137-47. 38. Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1155-9. 39. Doran M. AAO. Understanding and treating viral anterior uveitis. September 2009. www.aao.org/eyenet/article/viral-anterior-uveitis. Accessed July 29, 2021. 40. Centers for Disease Control and Prevention. Dengue. www.cdc.gov/dengue. Accessed July 29, 2021. 41. Lim WK, Mathur R, Koh A, et al. Ocular manifestations of dengue fever. Ophthalmology. 2004;111(11):2057-64. 42. Chan DPL, Teoh SCB, Tan CSH, et al. Ophthalmic complications of dengue. Emerg Infect Dis. 2006;12(2):285-9. 43. Jabs DA, Busingye F. Approach to the diagnosis of the uveitides. Am J Ophthalmol. 2013;156(2):228-36. |
