 |
A Modern Approach to Meibomian Gland Dysfunction
Learn how to effectively identify, diagnose and treat this condition.
By Kambiz Silani, OD
Release Date: November 15, 2020
Expiration Date: November 15, 2023
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss the cause of meibomian gland dysfunction.
- Describe the necessary clinical steps to help patients prevent gland dysfunction and ocular surface disease.
- Discuss the new imaging technologies that can help clinicians assess gland function.
- Review the diagnosis with patients and how treatment is changing their glands.
Target Audience: This activity is intended for optometrists engaged in the care of patients with meibomian gland dysfunction.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Kambiz Silani, OD, chief clinical director of Beverly Hills Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 70085-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Silani receives consulting fees from NuLids and fees for non-CME/CE services from Sight Sciences, Johnson and Johnson and Lumenis.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
|
Ocular Surface Health - Red Eye Remedies: New and Tried-and-True - How to Answer the “Why?” of Dry Eye - Artificial Tears: What Matters and Why |
Meibomian gland dysfunction (MGD), a well-known driver of ocular surface disease, can prove challenging for both clinicians and patients. To provide effective care, clinicians must understand the causes of this condition and the underlying mechanisms at play. This article provides a closer look at the meibomian glands and the pathophysiology of MGD. It also delves into the clinical steps that can help patients prevent dysfunction. New imaging technologies are valuable tools not only for disease assessment, but also patient education. The ability to properly guide the exam journey—from the diagnostic testing to treatment plan—is imperative for optimal outcomes and patient satisfaction.
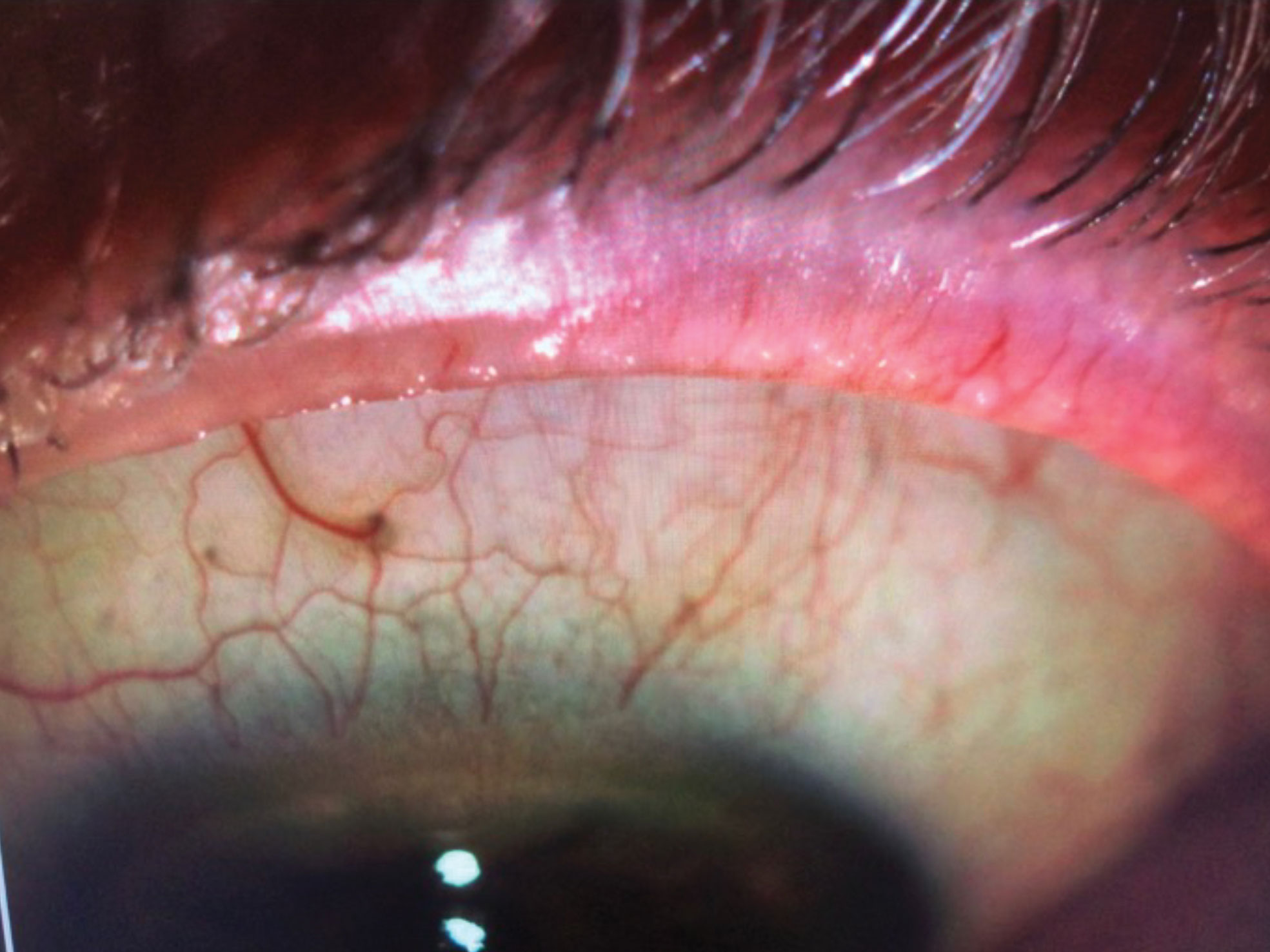 |
| IPL can help remove telangiectasia in the eyelid margins of MGD patients. Click image to enlarge. |
MGD Explained
Meibomian glands are sebaceous glands present within eyelids that secrete meibum, a compound made of lipids that form the superficial layer of the tear film. Meibum is delivered to the ocular surface, where it coats the aqueous layer and provides tear film stability and protection against microbial agents.1 In healthy individuals, there are approximately 25 to 40 glands in the upper eyelid and about 20 to 30 in the lower eyelid with lengths of about 5.5mm in the upper eyelids and 2mm in each of the lower eyelids.2
Proper functioning of the glands serves to: (1) minimize tear film evaporation, (2) enhance the tear film stability, (3) provide a smooth optical surface for the cornea and (4) seal the opposing lid margins during sleep.3
A number of different factors may contribute to dysfunction of meibomian glands, which leads to dry eye disease and its characteristic ocular surface inflammation.
Of the estimated 30 to 40 million Americans with dry eye disease, 86% have some form of MGD.4 This condition, defined as a chronic, progressive and diffuse abnormality of the meibomian glands, is commonly characterized by duct obstruction, static meibum, cystic dilation, abnormal gland secretion and eventual gland atrophy and dropout.1 Obstruction of the meibomian glands reduces delivery of meibum to the lid margin and may present with or without inflammatory features.5 Disruption of meibomian gland function negatively impacts both the quality and quantity of meibum secreted, which in turn affects ocular surface health through changes in tear film composition.1
In the early stages of MGD, anatomical changes are present even though patients are often asymptomatic. The lack of symptoms can cause delays in proper diagnosis and treatment. As the disease progresses, the tear film consistency and uniformity may be disrupted across the ocular surface, often leading to uncomfortable and sometimes intolerable symptoms.
Some of the most common ocular symptoms include eye fatigue, discharge, foreign body sensation, dryness, gritty or sticky sensation, eye pain, epiphora, itching, redness, light sensitivity, excessive blinking, blurred vision and a history of chalazion or hordeolum.1 One method to identify these symptomatic patients is by integrating a standardized dry eye questionnaire (e.g., SPEED, OSDI, DEQ-5).
If left untreated, the stagnant meibum could lead to gland structure damage in the form of gland recession and atrophy. Of note, when meibomian glands are treated in the early stages, it is possible to restore their function, potentially before recession and atrophy sets in.6
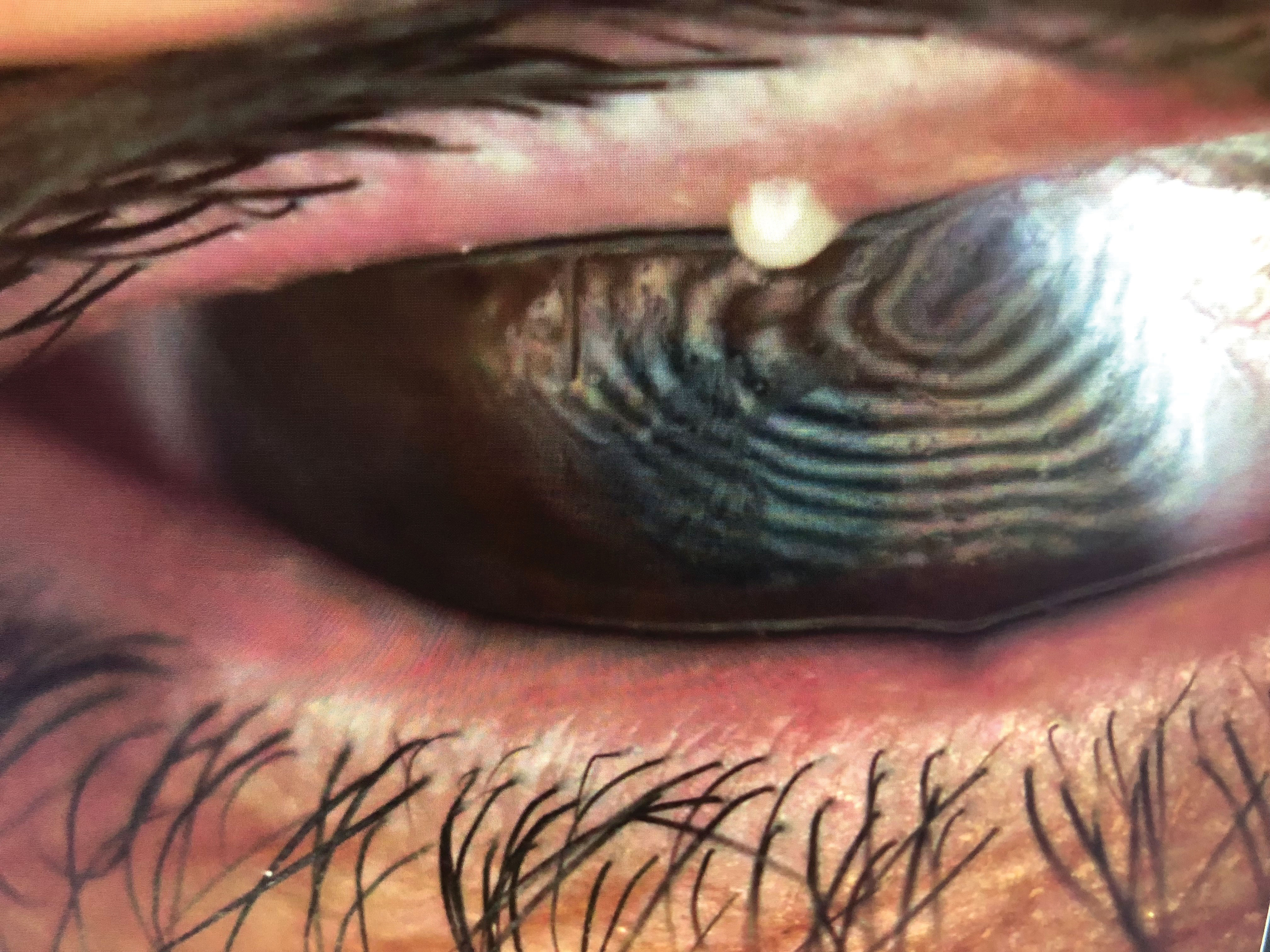
 |
| This patient presented with evidence of gland dropout and evaporative dry eye secondary to obstructive MGD. He was treated with BlephEx, immediately followed by LipiFlow. Non-invasive TBUT improved significantly pre- and post-LipiFlow treatment (left and right, respectively). Click image to enlarge. |
Gland Regeneration Controversy
Among eye care professionals, the general consensus was previously thought that meibomian gland atrophy was permanent. Recently, evidence suggests that some office-based treatments may modestly improve gland structure.7-9 Specifically, thermal pulsation therapy, intense pulsed light (IPL) and meibomian gland probing have some potential to reverse gland atrophy.
In one study, IPL treatment improved the OSDI symptom score, meibomian gland function and macrostructure and eyelid hygiene. IPL treatment also improved meibomian gland microstructure and decreased meibomian gland inflammation in MGD patients.7
Another study suggests that intraductal meibomian gland probing in patients with obstructive MGD could lead to growth and regeneration of gland tissue. Meibomian gland probing works, in part, by the introduction of a stainless steel wire instrument (76µm in diameter) to the gland orifice and into the ductal outflow tract that relieves obstruction and maintains patency of the outflow channel. Interestingly, meibography of post-probing patients revealed an increase in meibomian gland tissue area and regrowth of atrophied glands.8
Research looking into thermal pulsation therapy shows up to a 10% improvement in visual gland structure of the lower lids in 69% of patients with MGD.9 The potential to regenerate glands that have truncated is promising, although further research is necessary to reinforce these early findings and our understanding of true meibomian gland regeneration.
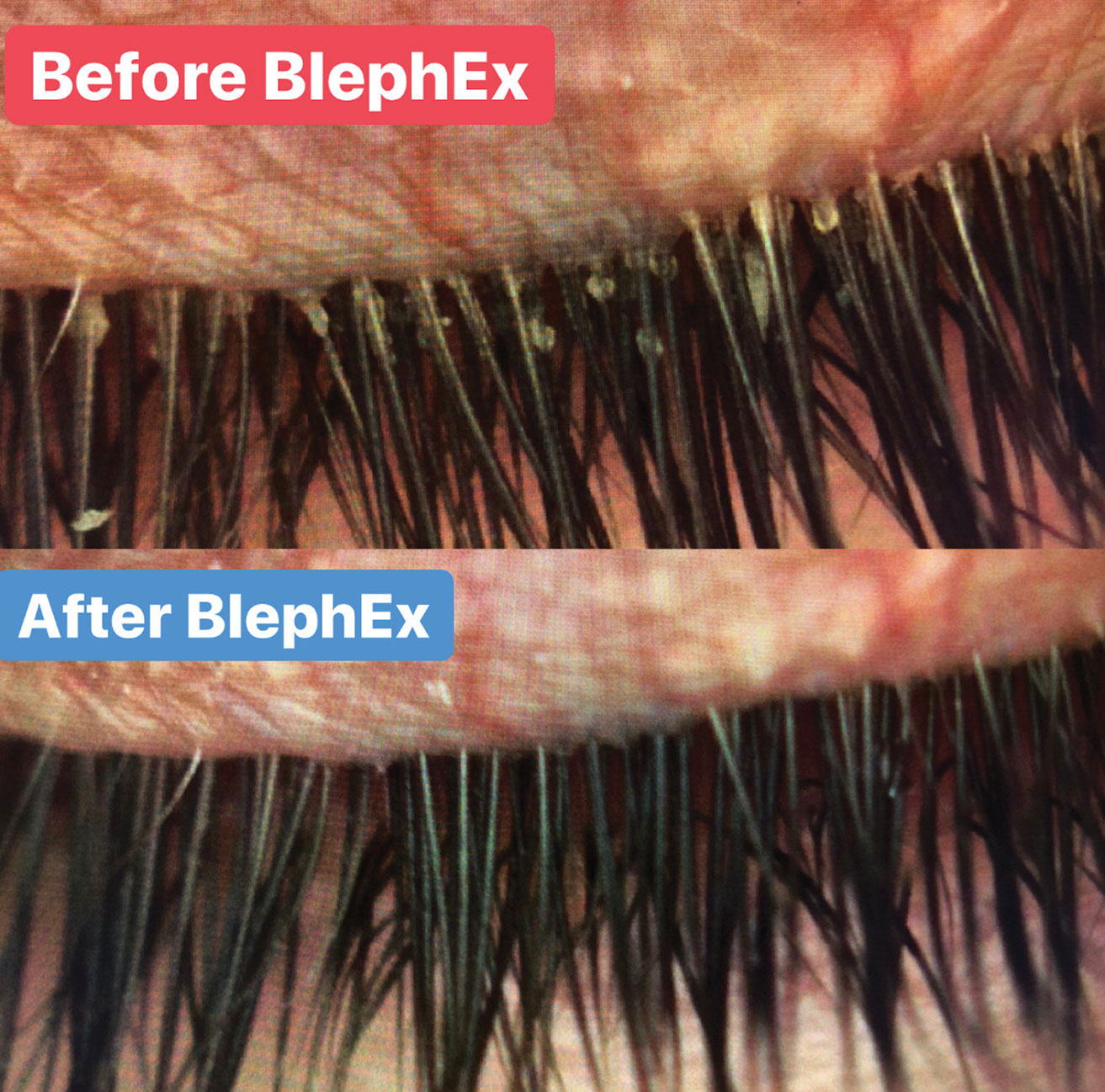 |
| Advances in the management of MGD include a number of office-based therapeutics, such as BlephEx. Lid debridement helps remove biofilm and debris and uncap the openings of the oil glands. Click image to enlarge. |
Leading Causes
There is an extensive list of risk factors for dry eye disease and MGD. When reviewing patient history, keep these major contributing factors in mind:
Hormone fluctuation. Among women with a hormonal imbalance, current research suggests that sex hormone levels may influence meibomian gland gene expression and lipid production.10
Cosmetics. Another risk factor is the improper application and removal of eye makeup. The regular use of eyeliner induces tear film instability and MGD.11 Additionally, common topical preservatives in cosmetics and topical drops may be toxic to the meibomian gland epithelial cells.12
Medications. Some drugs, such as antihistamines, antidepressants, birth control pills and blood pressure medicine, can cause MGD.13 For instance, glaucoma drops have potential adverse effects on the cornea and ocular surface. Specifically, prostaglandin analogs are associated with both a higher prevalence and severity of obstructive MGD.14 When looking at certain oral medications such as Accutane (isotretinoin), research shows this class of medication can alter the quality of the meibum or reduce the vitality of the glands as a whole.15,16
Digital devices. A notable risk factor for MGD is infrequent and improper blinking, especially when using digital devices, as this leads to stagnant oils and damage to the meibomian gland anatomy.17
Other causes. Research has uncovered a number of other risk factors, including:18-22
- increased age
- female gender
- certain medical conditions such as diabetes, rosacea and autoimmune conditions
- poor diet
- allergies
- laser eye surgery and cataract surgery
- contact lens overwear
- dry climate or low humidity
- windy conditions
- exposure to smoke
- bacterial/Demodex overgrowth
|
Billing Tips During the dry eye workup, external photography is considered billable when linked with an appropriate diagnosis such as corneal neovascularization, external hordeolum or anterior blepharitis secondary to Demodex infestation. Unfortunately, meibography, which was previously billable under external photography, now has its own level three CPT code and is no longer payable until the new code is considered a level one CPT code. Of the office-based treatments, only two have billable codes: punctal plugs and amniotic membranes. Unfortunately, neither of these address the obstruction, lid hygiene and ocular surface inflammation concerns. Although lid debridement, thermal expression and IPL devices do not have reimbursable codes, our patients recognize the importance of early detection and intervention with these treatments to preserve the gland structures and function. |
Diagnostics
Modern imaging and point-of-care testing elevate the patient care experience by providing valuable diagnostic metrics. A robust dry eye workup helps set a baseline, the characteristics of which include the following six diagnostic tests:
Evaluation of the eyelid anatomy. Lid margin assessment with a slit lamp or external photography can rule out vascular engorgement, plugged meibomian gland orifices, displacement of the mucocutaneous junction and overgrowth of Demodex/bacteria.23,24
Meibography. This is a useful tool to help clinicians grade the anatomy of the meibomian glands. There are two commonly used scales with a grading of 0 to 3 or 0 to 4, where 0 represents no disease and grades 3 and 4 represent end-stage disease with loss of more than 67% (grade 3 max scale) or more than 75% (grade 4 max scale) of the gland structure. These grading systems are useful to group patients by disease severity (normal, mild, moderate, severe).25
Tear film break-up time (TBUT). This is a standard, reliable and repeatable metric that determines which areas of the tear film are more susceptible to quicker evaporation and, therefore, have a higher potential for ocular surface dryness and damage.26 TBUT can be performed with fluorescein dye during slit lamp examination, while non-invasive TBUT can be performed with a specialized device that requires no drops or dyes.
Meibometry. When grading the function of the meibomian glands, evaluate the flow of the meibum with moderate digital pressure. This can be applied with a cotton swab or a meibomian gland expressor (Johnson & Johnson Vision). One commonly accepted grading system uses a scale of 0 to 3, where grade 0 represents no secretion of meibum (severe), grade 1 is inspissated, toothpaste-like consistency (moderate), grade 2 is cloudy, diffuse turbid fluid (mild) and grade 3 is clear, normal meibum.25
Inflammatory marker. Clinicians can assess matrix metalloproteinase-9 (MMP-9) levels using the MMP-9 test (InflammaDry, Quidel), a single-use, rapid-result test. When performing the in-office test, a tear film sample is collected and the results are displayed in 10 minutes as either positive or negative depending on whether the concentration of MMP-9 levels are greater or less than 40ng/ml. Awareness of the presence or absence of inflammation on the ocular surface aids in clinical decision making and the treatment plan.27
Tear osmolarity. TearLab is an easy-to-use, handheld diagnostic instrument that uses disposable, test cards to measure the solute concentration of tears. The results are considered abnormal, indicating a loss of homeostasis, when either eye has an osmolarity of greater than 300mOsm/L or there is a difference greater than 8mOsm/L between the eyes. Loss of meibum expression in patients with obstructive MGD leads to increased evaporation of water, increased tear osmolarity and symptoms of MGD.28
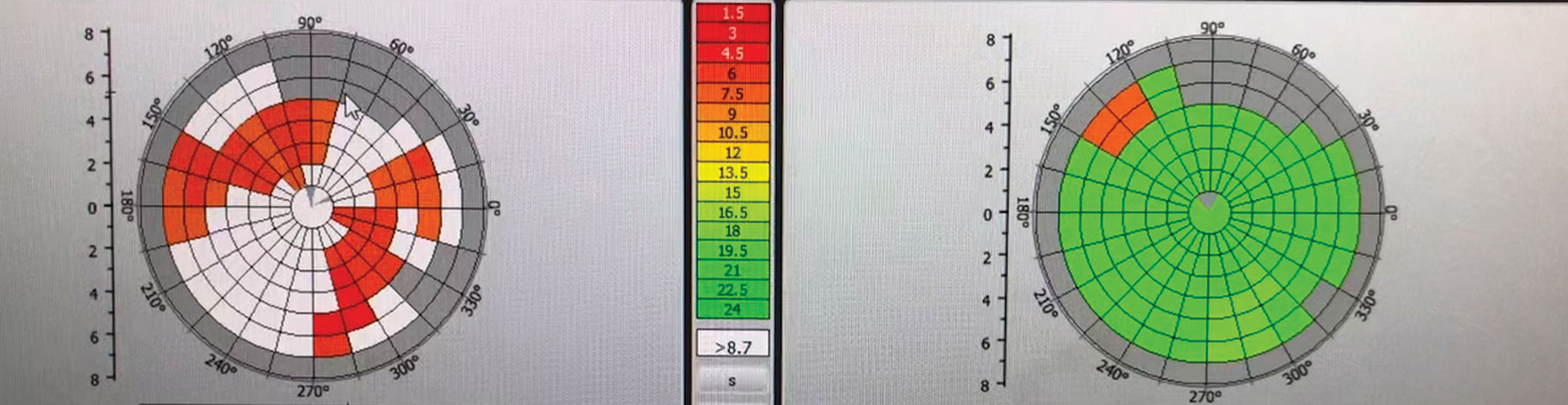
 |
| Imaging shows lid engorgement, telangiectasia and blocked meibomian glands with a milky, thick appearance (grade 1 on meibometry). Click image to enlarge. |
Office-based Treatments
Vast advances have been made in the management of MGD, leading to a number of new treatment options for an otherwise complex disease process. When using office-based therapeutics, consider a three-step approach: lid margin hygiene, removal of obstruction and reduction or elimination of inflammation. These three treatment categories are synergistic and most effective when performed earlier in the disease process, before patients reach end-stage MGD with significant gland atrophy and dropout.
Microblepharoexfoliation. Early biofilm may seal off the gland orifices and gradually fill the gland, mixing with meibum and leading to chronic lid disease and discomfort. Lid debridement can remove biofilm and debris and uncap the openings of the oil glands. There are two electronic debridement devices, BlephEx and AB Max (Myco Industries), as well as a new manual debridement technique with Zocular Eyelid System Treatment (ZEST, Zocular).
A recent study demonstrated that one ZEST session improved contact lens-related discomfort, doubled contact lens wear time, decreased usage of rewetting drops and reduced MMP-9 levels.29
Thermal expression options. These thermal modalities heat the meibum and melt the stagnant oils, allowing for proper expression and clearance. For patients with obstructive MGD, consider following lid debridement with thermal expression to maximize the therapeutic efficacy. Applying lid debridement pre-thermal expression removes surface biofilm and allows for easier outflow of meibum post-thermal expression. A number of office-based devices are available, including LipiFlow (Johnson & Johnson Vision), TearCare (Sight Sciences), iLux (Alcon), MiBo Thermoflo (MiBo Medical Group) and Thermal 1-Touch (Ocusoft).
LipiFlow treats the root cause of MGD. One study looked at the benefits of this treatment for contact lens wearers with MGD. Data shows that a single vectored pulsation treatment increased comfortable wearing time by approximately four hours per day.30
A single treatment with TearCare, a portable tool that addresses obstructive MGD, can provide a safe and successful improvement in signs and symptoms of dry eye disease in patients with MGD.
Researchers also compared the treatment with LipiFlow, where TearCare met non-inferiority treatment standards.31 In a randomized clinical trial there were no statistically significant differences between the iLux system and LipiFlow.32
The MiBo Thermoflo system and the Thermal 1-Touch device both heat and melt solidified oils that have become stuck inside the meibomian glands and should be followed immediately by manual gland expression. Researchers found that MiBo Thermoflo treatment resulted in a statistically significant improvement in the condition of the eyelids.33
To date, there are no studies on the Thermal 1-Touch device.
The MiBo Thermoflo requires a doctor, technician or staff member to apply the heat, while the Thermal 1-Touch device, similar to a trial frame, is placed on the patient and heats automatically.
Neither device has disposable options; therefore, they must be sterilized thoroughly prior to each patient use.
Intense pulsed light. This treatment addresses telangiectasia, lid swelling and inflammation. Through the use of photobiomodulation properties, IPL selectively targets abnormal blood vessels leaking inflammatory meditators, heats and liquifies meibum, eradicates Demodex and suppresses MMP-9 levels.34 Multiple studies show that IPL combined with gland expression provides significant improvement in objective and subjective findings.35 As a bonus, post-IPL patients may also notice skin rejuvenation effects such as reduction in sunspots, minimization of vascular lesions and stimulation in collagen growth, leading to improvement in fine lines and wrinkles.
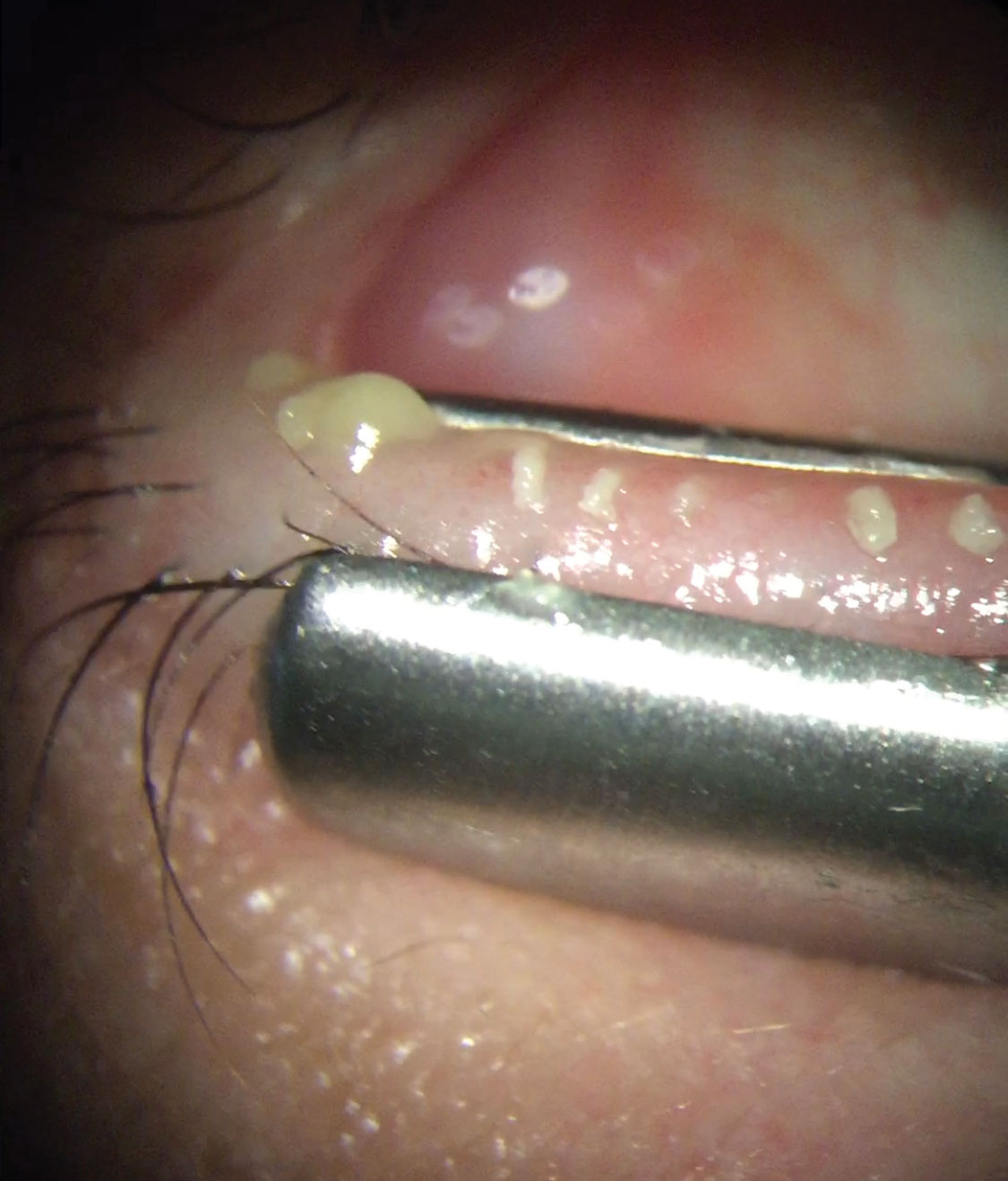 |
| Modern imaging and point-of-care testing, including meibometry, offers valuable diagnostic metrics and helps reveal the severity of MGD. Click image to enlarge. |
At-home Eye Care
Setting a customized home routine for MGD patients may enhance the effects of office-based treatment and allows for continued maintenance between follow-up visits. This can be analogous to a patient visiting their dentist, where a deep cleaning is performed while stressing the importance of maintaining proper dental hygiene between visits. Depending on your patient’s signs and symptoms of MGD, consider the following at-home routine:
Eyelid cleansers. Hypochlorous acid (HOCL) and tea tree oil are effective at targeting bacterial overgrowth and eliminating Demodex, respectively.31,32 HOCL is a powerful, natural compound that kills a broad spectrum of bacteria without altering the floral diversity on the periocular skin.36 It also successfully helps relieve chronic eye conditions, such as dry eye, blepharitis, MGD, contact lens intolerance and inflammation. Additionally, tea tree oil has proven effective in treating MGD associated with Demodex.37 Clean eyelid margins provide lasting relief and create healthier and happier MGD patients.
Warm compress therapy. Some patients will use a warm washcloth or other “do-it-yourself” methods of creating a pseudo-compress. These may be ineffective at properly maintaining and distributing the heat for a five- to 10-minute timeframe. Using an antimicrobial warm compress once or twice daily provides sustained, deep penetrating moist heat to the eyelids, leading to improvement of MGD.38
Omega fatty acids. Oral supplementation and consumption of omega-3 fatty acids can improve the meibum quality and expression.39 It is associated with a statistically significant improvement in tear osmolarity, TBUT, MMP-9 and OSDI symptom scores.40
Artificial tears. When considering lubricating drops for evaporative dry eye patients, clinicians should select ones with a higher viscosity to support abnormal lipid layer.
At-home debridement. The NuLids system (NuSight Medical) is a handheld device for at-home treatment of MGD and blepharitis. The oscillating action of the disposable tip cleans the lid margins and is designed to reduce the biomass load, remove collarettes, uncap blocked glands and stimulate production of meibum.
Lifestyle modifications. Discussing lifestyle adjustment is equally important to maximize success. Depending on various factors, some recommendations to consider for your MGD patients include increasing water intake, modifying diet, improving computer ergonomics and reviewing blink exercise techniques and proper cosmetic application and removal.
To provide comprehensive patient care, clinicians must understand MGD and its mechanisms. Additionally, imaging can be used to educate patients on the role of the meibomian glands and emphasize the importance of lid hygiene. This lays the foundation for success while motivating patients to collaborate with their clinician to create a treatment plan that aligns with their lifestyle and clinical goals.
Dr. Silani is the chief clinical director of Beverly Hills Optometry in Beverly Hills, Calif.
| 1. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20-S26. 2. Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-78. 3. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806. 4. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. 5. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243-51. 6. Kim S, Blackie CA, Korb DR. Restoration of meibomian gland function after treatment for meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2012;53(14):601. 7. Yin Y, Liu N, Gong L, et al. Changes in the meibomian gland after exposure to intense pulsed light in meibomian gland dysfunction (MGD) patients. Curr Eye Res. 2018;43(3):308-13. 8. Maskin SL, Testa WR. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2018;102:59-68. 9. Hura A, Epitropoulos A, Czyz C, et al. The potential effect of LipiFlow on meibomian gland structure: A preliminary analysis utilizing dynamic meibomian imaging. Invest Ophthalmol Vis Sci. 2018;59:935. 10. Versura P, Giannaccare G, Campos EC. Sex-steroid imbalance in females and dry eye. Curr Eye Res. 2015;40(2):162-75. 11. Prabhasawat P, Chirapapaisan C, Chitkornkijsin C, et al. Eyeliner induces tear film instability and meibomian gland dysfunction. Cornea. 2020;39(4):473-8. 12. Wang J, Liu Y, Kam WR, et al. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp Eye Res. 2020;196:108057. 13. Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851. [correction: J Ophthalmol. 2019:2989680]. 14. Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016:25(9):770-4. 15. Yeh, T. Effects of isotretinoin on meibomian glands. UC Berkeley. 2019. escholarship.org/uc/item/3mm9k54s. Accessed October 8, 2020. 16. Moy A, McNamara NA, Lin MC. Effects of isotretinoin on meibomian glands. Optom Vis Sci. 2015;92(9):925-30. 17. Sheppard AL, Wolffsohn JS. Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018;3(1):e000146. 18. Nien CJ, Massei S, Lin G, et al. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129(4):462-9. 19. Park Y, Hwang HB, Kim HS. Observation of influence of cataract surgery on the ocular surface. PLoS One. 2016;11(10):e0152460. 20. Kwan JT, Opitz DL, Hom MM, et al. Gender differences in a meibomian gland dysfunction-specific symptom questionnaire. Invest Ophthalmol Vis Sci. 2014;55:22. 21. WebMD. What is meibomian gland dysfunction? www.webmd.com/eye-health/meibomian-gland-dysfunction. Accessed October 8, 2020. 22. Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom. 2017;9:41-8. 23. Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015;15(Suppl 1):156. 24. Mudgil P, Borchman D, Yappert MC, et al. Lipid order, saturation and surface property relationships: A study of human meibum saturation. Exp Eye Res. 2013;116:79-85. 25. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006-49. 26. Tsubota K. Short tear film breakup time–type dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES64-70. 27. Lanza NL, Valenzuela F, Perez VL, et al. The matrix metalloproteinase 9 point-of-care test in dry eye. Ocul Surf. 2016;14(2):189-95. 28. Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10:286-90. 29. Narayanan S, Kasraie N, Stewart M, et al. Lid margin debridement improves contact lens discomfort caused by meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2019;60(9):6368. 30. Blackie CA, Coleman CA, Nichols KK, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction, increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2019;12:169-83. 31. Loh J, Trattler WB, Dhamdhere KP, et al. A novel, targeted, open eye, thermal therapy and meibomian gland clearance in treatment of dye eye: A randomized control trial (OLYMPIA). American Society of Cataract and Refractive Surgery (ASCRS) Virtual Annual Meeting. May 16, 2020. 32. Tauber J, Owen J, Bloomenstein M, et al. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: A randomized clinical trial. Clin Ophthalmol. 2020;14:405-18. 33. Connor CG, Narayanan S, Miller WL. The efficacy of MiBo Thermoflo in treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2016;57(12):5678. 34. Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophtalmol. 2017;11:1167-73. 35. Dell SJ, Gaster RN, Barbarino SC, et al. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817-27. 36. Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-14. 37. Maher TN. The use of tea tree oil in treating blepharitis and meibomian gland dysfunction. Oman J Ophthalmol. 2018;11(1):11-15. 38. Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29(2):96-9. 39. Liu Y, Kam WR, Sullivan DA. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea. 2016;35(8):1122-6. 40. Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-91. |
